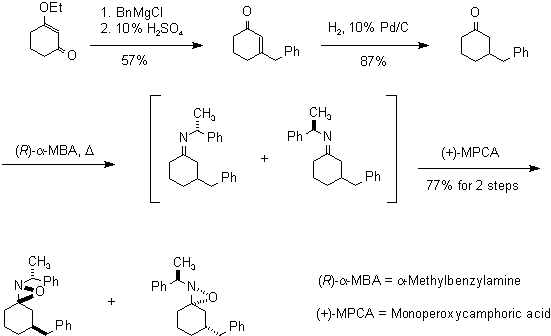
Racemic 3-benzylcyclohexanone is the precursor in this stereoselective synthesis of spirocyclic oxaziridines. It is condensed with (R)-a-methylbenzylamine which is oxidized with a bulky oxidizing agent such as monoperoxycamphoric acid to yield a mixture of primarily two oxaziridines.

The formation of oxaziridines is a stereoselective process
explained by the equatorial approach of the oxidizing agent toward the
imine thus producing the stereoisomeric adducts.