| [Related articles/posters: 028 116 012 ] |

Beta- and gamma-lactams and lactones are synthetically useful intermediates in synthesis. Many methods have been developed to prepare these ring systems: for example [2+2] cycloaddition of ketenes to either imines or aldehydes is a well known method for the preparation of beta-lactams and lactones and the cyclization of 4-pentenoic acids has been used to prepare gamma-lactones.1
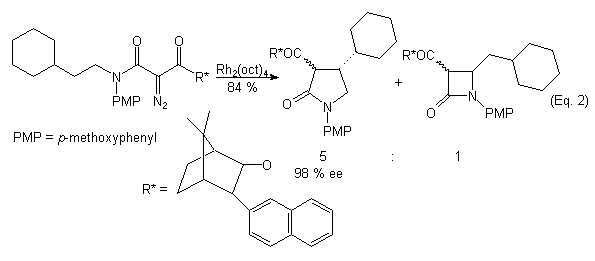
The intramolecular rhodium(II) carbenoid C-H insertion reaction of acyclic diazocarbonyl compounds has been shown to be a facile method for the construction of heterocyclic ring systems.2 Recently, attention has been directed towards the preparation of chiral non-racemic heterocycles via asymmetric Rh(II) carbenoid C-H insertion reactions.3 Two approaches have been described. The first involves the use of homochiral dirhodium(II) catalysts to effect the asymmetric C-H insertion in diazoamides and/or diazoesters (Eq. 1).3,4 The second approach involves the Rh(II)-catalyzed asymmetric C-H insertion of diazoamides possessing chiral auxiliary groups (Eq. 2).5
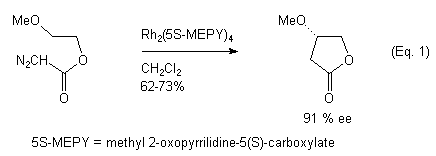
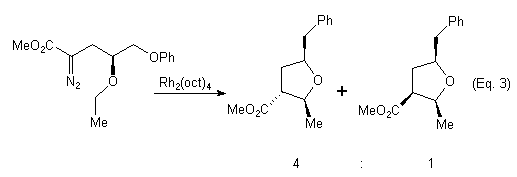
In connection with our ongoing interest in asymmetric C-H insertion reactions of diazoamides and diazoesters, we have investigated the use of 1,n-relative induction as a way to control the diastereoselectivity of the C-H insertion. Taber et al. have reported the synthesis of substituted tetrahydrofurans,6a (Eq. 3) and cyclopentanes6b (Eq. 4) in which 1,3-relative induction was exploited for controlling the diastereoselectivity of the C-H insertion. In our studies we have examined the Rh(II)-catalyzed C-H insertion reactions of compounds of type 1 (Eq. 5). In our system, the pre-existing , configurationally defined stereocenter is located exocyclic to the forming ring. Our objectives are two fold: 1) To determine the influence of steric and/or electronic effects on the regioselectivity of the reaction and 2) To ascertain the extent of 1,2-relative induction in the formation of gamma-lactams and lactones, and the extent of 1,3-relative induction in the formation of beta-lactams and lactones. We report our preliminary findings here.
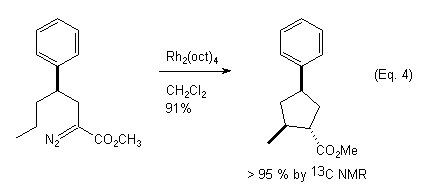
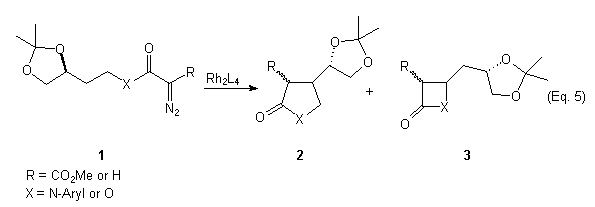
Preparation of Diazocarbonyl Compounds
Preparation of the diazo compounds starting from (S)-malic acid was accomplished through standard chemical manipulations and is shown in Scheme 1. We found that the conversion of ester 7 to the alcohol 13 was best performed with Cp2Ti-PMHS (PHMS= poly(methylhydro)siloxane).7 The reduction of 7 using either LiAlH4 or BH3.SMe2 gave complex mixtures of products. Another less direct route to the ketal alcohol 13 was initially used.8 The (2S)-1,2,4-butatriol obtained by reduction of (S)-malic acid with BH3.SMe2 and trimethyl borate was ketalized with acetone and p-TsOH to give a 9:1 mixture of 5- and 6-membered ketals. The ketal mixture was derivatized with 3,5-dinitrobenzyl chloride and fractionally crystallized to give pure 5-membered ketal which was hydrolyzed to give pure 13. It was found that the Cp2Ti-PMHS route was shorter and gave 13 in higher yield.
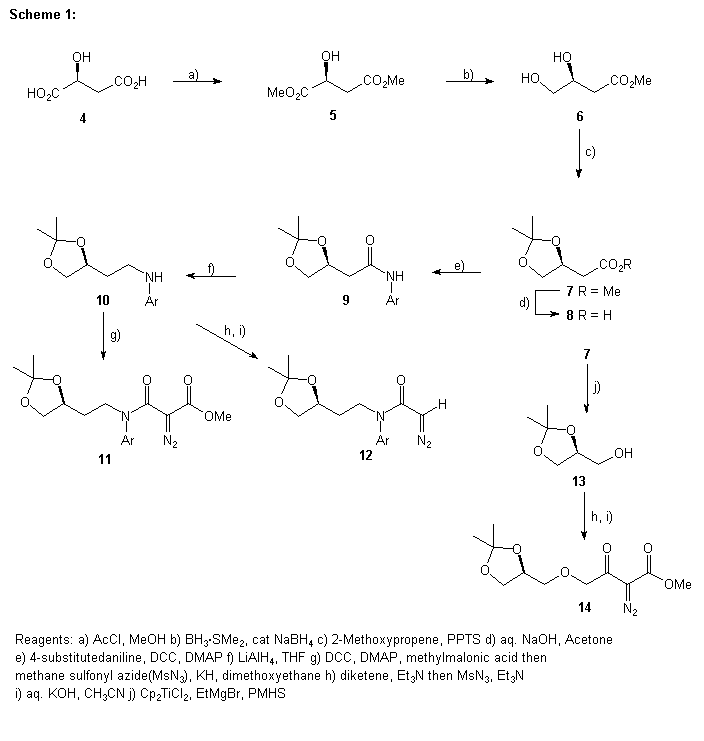
Rhodium(II) Catalyzed Reactions of Compound 11
Using diazocarbonyl 11 the Rh(II)-catalyzed C-H insertion reactions were carried out at a concentration of 0.10 M with 1 to 5 mole percent of catalyst. Studies were conducted and the regioselectivity was addressed by varying the solvent, temperature, catalysts and mode of addition, our results are summarized in Table 1.
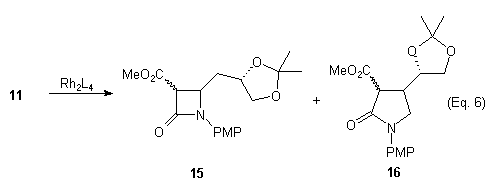
Table 1: Results of Rhodium(II)-Catalyzed C-H insertion of 11
|
. |
Catalyst | Yield
(%) |
Temperature
(oC) |
Ratio 15 : 16
(Beta:Gamma) |
Solvent |
| 1 | Rh2(OAc)4 | 75.2 | 23 | 1.0 : 1 | CH2Cl2 |
| 2 | Rh2(OAc)4 | 64.0 | 40 | 1.5 : 1 | CH2Cl2 |
| 3 | Rh2(oct)4 | 64.6 | 23 | 1.7 : 1 | CH2Cl2 |
| 4 | Rh2(OAc)4 | 81.9 | 23 | 1.2 : 1 | C6H6 |
| 5 | Rh2(OAc)4 | 56.2 | 80 | 2.4 : 1 | C6H6 |
| 6 | Rh2(Piv)4 | 56.3 | 80 | 5.0 : 1 | C6H6 |
| 7 | Rh2(acam)4 |
. |
80 | Ar. Substitution, H2O
insertion, Polar products |
C6H6 |
The trends observed in the regioselectivity studies are:
a) With CH2Cl2 as the solvent more gamma-lactam, 16 was obtained
b) An increased reaction temperature lead to reduced yields and a higher beta : gamma ratio
c) The more sterically demanding catalyst, Rh2(Piv)4, lead to much higher beta : gamma ratio
d) An electronically selective catalyst, Rh2(acam)4, leads to undesired products
We also found that for compound 11 slow addition made no difference in the regioselectivity or yield of the reactions.
The products obtained from the reactions were mixtures of possibly four diastereomers. The gamma-lactams 16 appeared to have only two components while the beta-lactams 15 gave a very complex 1H NMR indicating more diastereomers. To aid in the NMR anaylsis of the gamma-lactam 16, it was decarboxylated (aq. DMSO, NaCl). As expected, beta-lactam 15 gave a complex mixture under the same conditions. To avoid decarboxylation of the beta-lactams 15 it was decided to prepare the unsubstituted diazo 17a. When compound 17a was treated with Rh(II) catalysts no lactam products were isolated and only the oxindole 18a was formed. This was not unexpected because it is known that diazo compounds of type 12 and 17a prefer to undergo aromatic substitution over C-H insertion. After seeing the work of Anada and Hashimoto we decided to use p-nitrophenyl instead of p-methoxyphenyl as the N-protecting group.4 We prepared diazoamide 17b and subjected it to Rh(II)-catalyzed C-H insertion. A mixture of products was obtained and the IR and 1H NMR indicated that the corresponding oxindole 18b was not formed.
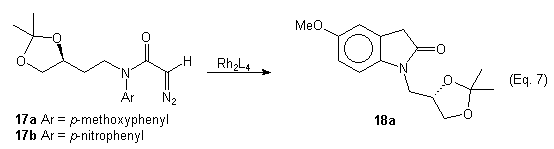
Rhodium(II)-Catalyzed Reaction of Compound 14
The diazoester 14 when subjected to a Rh(II) catalyst initially gave very low yields of the lactones and a large amount of polar by-products (H20 insertion and dimerization). However; when the reaction apparatus was thourghly flame dried under a stream of nitrogen and the substrate was added slowly (syringe pump) to the catalyst the amount of polar by-products was dramatically decreased.
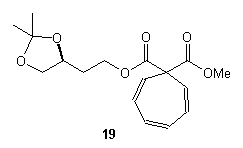
The ratio of beta : gamma lactones ranged from 6.1:1 to 3.0:1 with Rh2(OAc)4 as the catalyst while Rh2(acam)4 and Rh2(Piv)4 catalysts were found unsuitable because much more dimerization and H2O insertion was observed. When benzene was used as the solvent the cycloheptatriene 19 was obtained as the major product.
Assignment of Configuration at the New Stereogenic Center
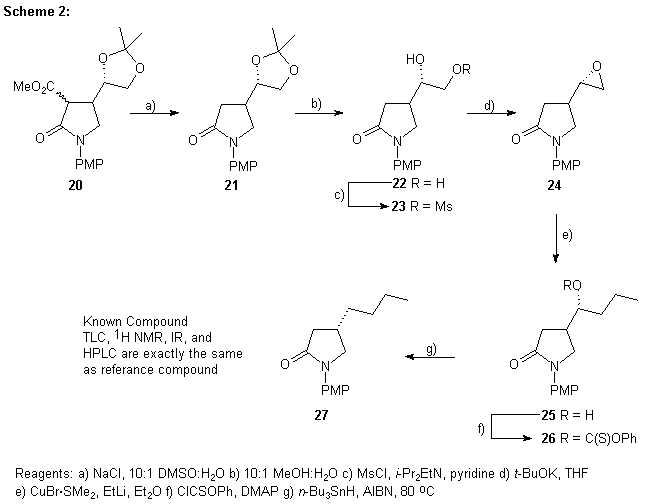
We initially focused on determining the sense of induction in the formation of the gamma-lactam 16. For 16 the sense of induction was determined by two methods. First compound 16 was converted to the known lactam 27, as summarized in Scheme 2, via a series of standard chemical manipulations. Great care was taken to avoid racemization at C-4 and not to remove any of the minor diastereomer so the extent of the 1,2-relative induction could be determined. Compound 27 had previously been prepared in our lab5 and was compared directly to the sample prepared from the gamma-lactam 16. The HPLC analysis (Chiralcel OB®, 70:30 hexane:iso-propanol) indicated that the major enatiomer was in fact the (4S)-gamma-lactam 27 and the ee. was found to be 75%.
A second independent confermation of the sense of induction was provided by the results of an x-ray analysis of the gamma-lactam 28 that was prepared from (R)-malic acid following the same steps for the preparation of 16. The results of the analysis indicated that the new stereocenter has the same sense of induction as the inducing center. Therefore in 1,2-relative induction, the new stereocenter has the S configuration if the inducing center is S, and R if the inducing center has the R configuration.
Results of our work show that good levels of 1,2-relative induction can be achieved with compounds of type 1 and that the sense of induction in these systems can now be predicted. The regioselectivity of these systems preferentially forms beta-lactams and lactones and can be controlled by reaction conditions. Future studies are aimed at improving on the diastereoselectivity of the C-H insertion reactions.
We are grateful to NSERC, Canada and the University of Regina for financial support.
DDM thanks the Faculty of Graduate Studies and the Department of Chemistry for scholarship support.
1) Collins, I. Comtemporary Organic Synthesis, 1997, 4, 281.
2) (a) Padwa, a.; Weingarten, D. Chem. Rev. 1996, 96, 223. (b) Ye, T.; Mckervey, A. Chem. Rev. 1994, 94, 1091. (c) Doyle, M. P. Chem. Rev. 1986, 86, 919.
3) (a) Doyle, M. P.; Forbes, D. C. Chem. Rev. 1998, 98, 911. (b) Doyle, M. P. Aldrichimica Acta. 1996, 29, 3.
4) Anada, M.; Hashimoto, S. Tetrahedron Lett. 1998, 39, 79.
5) (a) Wee, A. G. H.; Liu, B-S. Tetrahedron Lett. 1996, 37, 145. (b) Wee, A. G. H.; Liu, B-S.; Mcleod, D. D. J. Org. Chem. in press.
6) (a) Taber, D. F.; Song, Y. J. Org. Chem. 1996, 61, 6706. (b) Taber, D. F.; You, K. K.; Rheingold, A. L. J. Am. Chem. Soc. 1996, 118, 547.
7) Barr, K. J.; Berk, S. C.; Buchwald, S. L. J. Org. Chem. 1994, 59, 4323.
8) (a) Meyer, A. I.; Lawsom, J. P. Tetrahedron Lett. 1982, 23, 4883. (b) Hanessian, S.; Ugolini, A.; Dube, D.; Glamyan, A. Can. J. Chem. 1984, 62, 2146.