| [Related articles/posters: 005 050 033 ] |
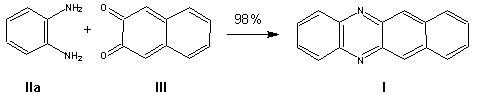
The solid-phase method was also used for the oxidation-reduction condensation of paraformaldehyde with some aromatic 1.2-diamines lla-llc, leading to N-methylbenzimidazoles (IVa-IVc). Using the standard procedure [3], N-methylbenzimidazoles IVa and IVb are obtained by heating formaldehyde with the corresponding diamine lla or llb in a mixture of ethanol and hydrochloric acid [3].
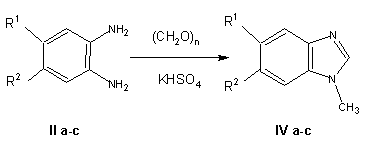
In the case of N-methyl-5-chlorobenzimidazole IVb, the solid-phase synthesis leads to the same products as in the reaction in solution, but the corresponding procedure is more convenient and suitable for industrial use since it permits the use of avialable paraformaldehyde and eliminates the need for heating and the use of readily inflammable ethanol and agressive hydrochloric acid. Naphtho derivative IVc could not be obtained in solution according lo the procedure of Ellis and Jones (3] since the active site 1 in the naphthalene fragment, which is additionally activated by two amino groups, probably is readily hydroxymethylated by paraformaldehyde, which leads to the formation of a polymeric product similar to phenol-formaldehyde resin. However, the use of the solid-phase method led to the synthesis of N-methyinaphthimidazole IVc.
Thus, the use of the solid-phase method for the synthesis of heterocyclic compounds may significantly simplify preparation of such compounds and provide for the synthesis of derivatives, which cannot be obtained in solution.
TABLE 1. Comparison of the Synthesis of Benzimidazolcs IV by Different Methods (%, yield)
| Compound | Substituents | Standard method | Solid-phase synthesis* |
| IVa | R1=H, R2=H |
|
|
| IVb | R1=Cl, R2=H |
|
|
| IVc | R1, R2 = CH=CH-CH=CH |
|
|
EXPERIMENTAL
The starting reagents were subjected in the solid state to different types of mechanical treatment: 1) grinding in a mortar. 2) synthesis in an SVM-0.4 vibrational ball mill (120 cm3 chamber volume, 24 Hz rotation frequency, 1-5 mm vibration amplitude), or 3) synthesis in a 20-cm3 plastic chamber containing steel beads and activated by a VP mixer. The PMR spectra were taken on a T-60 spectrometer at 60 MHz using HMDS as the internal standard.
Benzo[b]phenazine. A mixture of 3.16g (0.02 mole) 2,3-naphthoquinone III and 2.16g (0.02 mole) o-phenylenediamine lIa was treated in an SVM-0.4 vibrational mill for 10 min at 25-30íC to give 4.5 g (98%) benzo(b)phenazine as a yellow powder, mp 142-143 oC (142.5 oC [4]).
1-Methylbenzimidazole (IVa). A. A mixture of 0.1 g (0.93 mmole) o-phenylenediamine lIa. 0.1 g (3.3 mmoles) paraformaldehyde, and 0.45 g (3.3 mmoles) potassium hydrosulfate was stirred in a VP mixer with 1 g sodium sulfate using steel beads as the working element for 4 h and then treated with 5 ml 5% aq. sodium hydroxide. The mixture was extracted with three 3-ml portions of methylene chloride and dried over sodium sulfate. The solvent was evaporated. The residue was dissolved in 1 ml methylene chloride and subjected to chromatography on an alumina column with an upper layer of silica gel using 10:1 methylene chloride - 2-propanol as the eluent to obtain 0.047 (36%) 1-methylbenzimidazole IVa as an oil. PMR spectrum in CDCl3 3.7 (3H. s, CH3), 7.0-8.0 (5H, m. arom). Lit. mp 33 oC [5].
B. The synthesis was carried out analogously to procedure A but solid silica gel particles were used as the working element in the vibration treatment to give 0.06 g (45o) 1-methylbenzimidazole identical lo the sample obtained in procedure A.
C. A mixture of o-phenylenediamine lIa. parafonnaldehyde, potassium hydrosuifate, and sodium sulfate in the amounts indicated in procedure A was maintained in a porcelain mortar for 48 h with intermittent grinding with a pestle. The mixture was treated as in procedure A to give 0.04 g (30%) 1-methylbenzimidazole IVa identical to the sample obtained in procedure A.
1-Methyl-5-clorobenzimidazole (IVb). A. A mixture of 0.14g (1mmole) 4-chloro-1.2-diaminobenzene, 0.1g (3.3 mmoles) paraformaldehyde, 0.34 g (2.5 mmoles) potassium hydrosulfate. and 1 g sodium sulfate was stirred in a VP mixer using steel balls as the working element for 4 h and treated as in the above procedures io give 0.06 g (36%) 1-methyl-5-chlorobenzimidazole IVb as an oil. PMR spectrum in CCI4: 3.8 (3H. s. CH3, 7.0-8.0 (5H, m, arom). Lit mp 23-24 oC (3].
B. The synthesis was carried out analogously to procedure A but using solid silica gel particles as the working element and then maintaining the mixture for 96 h to give 0.066 g (40%) 1-methyl-5-chlorobenzimidazole IVb identical to the sample obtained in procedure A.
C. A mixture of 4-chloro-l,2-diaminobenzene llb, paraformaldehyde, potassium hydrosulfate, and sodium sulfate in amounts indicated in procedure A was maintained in a porcelain mortar for 24 h with intermittent grinding using a pestle am treated analogously to procedure A to give 0.085 g (52%) 1-methyl-5-chlorobenzimidazole IVb identical to the sample obtained in procedure A.
1-Methylnaphth[2,3-d]imidazole (IVc). A. A mixture of 0.195 g (1.23 mmole) 2.3-diaminonaphthalene, 0.11 g (3.75 mmoles) paraformaldehyde, 0.51 g (3.75 mmoles) potassium hydrosulfate. and 1 g sodium sulfate was treated in a VP mixer with steel balls as the working element for 3 h and separated as described above (to give 0.04 g (18%) 1-methyl-naphth(2,3-d)imidazole IVc. mp 146-148 oC (after sublimalion at 160-180 oC (15 mm Hg)]. mp 154 oC (from pelroleum ether). Lit. Bp 158 oC (from octane) (6). PMR spectrum in CDCl3 3.86 (3H, s, CH3), 7.1-8.3 (7H, m, arom).
B. The synthesis was carried out analogously to procedure A but using silica gel particles as the working element, mainained for 24 h, treated in VP mixer with steel balls for 2 h. maintained for two months, and then treated as in procedure A io give 0.033 g (15%) 1-methylnaphth[2,3-d]imidazole IVc identical lo the sample obtained in procedure A.
C. A mixture of 2,3-diaminonaphthalene IIc, paraformaldehyde, potassium hydrosulfate, and sodium sulfate in the amounts indicated in procedure A was maintained in a porcelain mortar for 24 h with intermittent grinding using a pestle, and then treated analogously to procedure A to give 0.05 g crude product, which upon sublimation at 160 oC (15 mm Hg) gave 0.022 g (10%) 1-methylnaphth[2,3-d]imidazole (IVc) identical to the sample obtained in procedure A.
This study was carried out with the support of the Russian Fund for Basic Research (Grant No. 96-03-33250).
REFERENCES
1. F. Toda, Syniett., No. 3, 303 (1993).
2. N. S. Enikolopyan, Zh. Fiz. Khim., 63. 2289 (1989).
3. G. P. Ellis and R. T. J. Jones. J. Chem. Soc., Perkin Trans. I, No. 10, 903 (1974)
4. C. B. Riolo and E. Marcon, Ann. Chim., 46. 528 (1956).
5. 0. Fisher and H. Wreszinski, Ber.. 85, 2711 (1892).
6. Beilstein. Vol. 23(5), No. 8, p. 203.