| [Related articles/posters: 034 077 010 ] |
compounds |
IVa |
IVb |
IVc |
IVd |
IVe |
reaction time [min.] |
5 |
4.5 |
2 |
2 - 3.5 |
1.5 - 2 |
yields [%] |
45.9 |
28.2 |
56 |
78 - 81 |
81 |
melting point [C] |
215-217 |
171-173 |
242-245 |
253-256 |
294-297 |
compounds |
IVf |
IVg |
IVh |
IVi |
IVj |
reaction time [min.] |
5b |
3.5a |
7b |
2b |
8b |
yields [%] |
45b |
79.5a |
52b |
53b |
61b |
melting point [C] |
231-233 |
255-258 |
239-242 |
174-177 |
229-232 |
compounds |
IVk |
IVl |
IVm |
IVn |
IVo |
reaction time [min] |
10 |
3 |
15 |
12 |
10 |
yields [%] |
62 |
51 |
38 |
41 |
42 |
melting point [C] |
221-223 |
175-177 |
275-278 |
277-279 |
203-205 |
Table 2. Characterisation of the prepared compounds IVa -
IVg.
R1 |
R2 |
R3 |
Formula |
calc. / found |
||||||
Mr [g.mol-1] |
%C |
%H |
%N |
%S |
%Br |
%Cl | ||||
IVa |
H |
H |
H |
C25H18BrNO2S |
63.03 |
3.81 |
2.94 |
6.73 |
16.77 |
|
IVb |
CH3 |
H |
H |
C26H20BrNO2S |
63.68 |
4.11 |
2.86 |
6.54
|
16.30 |
|
IVc |
Cl |
H |
H |
C25H17BrClNO2S |
58.78 |
3.35 |
2.74 |
6.
28 |
15.64 |
6.94 |
IVd |
Br |
H |
H |
C25H17Br2NO2S |
54.08 |
3.09 |
2.52 |
5.77 |
28.78 |
|
IVe |
NO2 |
H |
H |
C25H17BrN2O4S |
57.59 |
3.28 |
5.37 |
6.15 |
15.33 |
|
IVf |
H |
OH |
H |
C25H18BrNO3S |
60.98 |
3.68 |
2.84 |
6.51 |
16.23 |
|
IVg |
CH3 |
CH3 |
H |
C27H22BrNO2S |
64.29 |
4.40 |
2.78 |
6.36 |
15.84 |
Table 2. Continued. Characterisation of the prepared compounds
IVh - IVj and V.
R1 |
R2 |
R3 |
Formula |
calc. / found |
||||||
Mr [g.mol-1] |
%C |
%H |
%N |
%S |
%Br |
%Cl | ||||
IVh |
H |
OH |
OH |
C25H18BrNO4S
|
59.06 |
3.57 |
2.76 |
6.31 |
15.72 |
|
IVi |
Cl |
H |
CH3 |
C26H19BrClNO2S |
59.50 |
3.65 |
2.67 |
6.11 |
15.22 |
6.75 |
IVj |
OH H H |
C29H20BrNO2S |
66.16 |
3.83 |
2.66 |
6.09 |
15.18 |
|||
V |
H H H |
C25H20BrNO3 S |
60.67 |
4.04 |
2.83 |
6.47 |
16.18 |
|||
532.4519 |
60.87 |
3.85 |
2.54 |
6.70 |
16.50 |
Table 2. Continued. Characterisation of the prepared compounds
IVk - IVo.
R1 |
R2 |
R3 |
Formula |
calc. / found |
|||||
Mr [g.mol-1] |
%C |
%H |
%N |
%Br |
%Cl | ||||
IVk |
H |
H |
H |
C25H18BrNO3 |
65.23 |
3.94 |
3.04 |
17.36 |
|
IVl |
CH3 |
H |
H |
C26H20BrNO3
|
59.34 |
3.83 |
2.66 |
15.18 |
|
IVm |
Cl |
H |
H |
C25H17BrClNO3 |
60.69 |
3.46 |
2.83 |
16.15 |
7.16 |
IVn |
Br |
H |
H |
C25H17Br2NO3 |
55.69 |
3.18 |
2.60 |
29.64 |
|
IVo |
H |
OH |
H |
C25H18BrNO4 |
63.04 |
3.81
|
2.94 |
16.77 |
Tab. 3. 1H NMR spectra [delta] (ppm) (CF3COOH - d,
80 MHz)
(ppm ) | |
III |
3.3 (s, 3H, CH3); 6.13 (s, 2H, CH2); 7.37 (s, 5H, Ph); 7.76 - 7.88 (m., 2H, H-arom.); 8.49 - 8.61(m., 2H, H-arom.). |
IVa |
6.178 (s, 2H, CH2); 7.466 (s, 5H, Ph); 7.871-8.472 (m, 9H, H-5-9, H-11-14); 8.811 (s, 1H, H-2); 9.130 (d, 3J=15.38 Hz, 1H, H-10) |
IVb |
2.590 (s, 3H, CH3); 6.120 (s, 2H, CH2); 7.270-7.508 (m, 5H, Ph); 7.597-8.222 (m, 8H, H-5,7-9,11-14); 8.768 (s, 1H, H-2); 9.026 (d, 3J=15.38 Hz, 1H, H-10) |
IVc |
6.111 (s, 2H, CH2); 7.307-7.505 (m, 5H, Ph); 7.761-8.326 (m, 8H, H-5, 7-9, 11-14); 8.744 (s, 1H, H-10); 9.008 (d, 3J=15.38 Hz, 1H, H-10) |
IVd |
6.145 (s, 2H, CH2); 7.420 (s, 5H, Ph); 7.688-8.463 (m, 8H, H-5,7-9,11-14); 8.872 (s, 1H,H-2); 8.969 (d, 3J=15.38 Hz, 1H, H-10) |
IVe |
6.172 (s, 2H, CH2); 7.435 (s, 5H, Ph); 7.908-8.344 (m, 6H, H-8,9,11-14); 8.882 (d-d, J1=15.38 Hz, J2=2.44 Hz, 1H, H-7); 8.930 (s,1H, H-2); 8.989 (d, 3J=15.38 Hz, 1H, H-10); 9.281 (d, J=2.44 Hz, 1H, H-5) |
IVf |
6.120 (s, 2H, CH2); 7.221-7.420 (m, 7H ); 7.841- 8.301 (m, 7H ); 8.710 (s,1H, H-2); 9.004 (d, 3J= 15.38 Hz, 1H, H-10) |
IVg |
2.340 (s, 3H, CH3); 2.532 (s, 3H, CH3); 6.125 (s, 2H, CH2); 7.235 (s, 5H, Ph); 7.659-8.521 (m, 7H, H-5, 8, 9, 11-14); 8.865 (s, 1H, H-2); 9.105 (d, 3J=15.38 Hz, 1H, H-10) |
IVh |
6.004 (s, 2H, CH2); 7.209-7.578 (m, 7H); 7.957-8.384 (m, 7H); 8.979 (s, 1H, H-2); 10.251 (s, 1H, OH) |
IVi |
2.584 (s, 3H, CH3); 6.141 (s, 2H, CH2); 7.447 (s, 5H, Ph); 7.871-8.323 (m, 7H,H- 5,7,9, 11-14); 8.817 (s, 1H, H-2); 9.037 (d, 3J=15.62 Hz, 1H, H-10) |
IVj |
6.148 (2H, s,CH2); 7.444 (5H, s, Ph); 7.650-8.393 (10H, m,); 8.775 (1H, s, H-2); 8.869 (1H, d, J=15 Hz); 9.769 (1H, d, J=9 Hz) |
Va |
6.15 (s, 2H); 7.39 (s, 5H, H-Ph); 7.61 (t, 1H, H-5); 7.81 - 7.89 (m, 4H, H-Bt-het.); 8.06 (d, 1H, H-9, 3J=15Hz); 8.702 (d, 1H, H-10, 3J=15Hz); 8.19 (dd, 1H, H-6, 3J=8Hz, 4J=1.43Hz); 8.32 (d, 1H, H-8, 3J=8Hz); 8.50 (dd, 1h, H-7, 3J=8Hz, 4J=1.44Hz); 9.174 (s, 1H, H-2) |
aDMSO
- d6, 300 MHz (Varian GEMINI 2000)
Table 4. Enthalpy reaction ([Delta]H), for preparing salts IV,
compounds V and heats of formation ([Delta]Hf) for
compounds V and VI (in Kcal mol-1)
R1 |
[Delta]H(IV) |
[Delta]H(V) |
[Delta]Hf(V) |
[Delta]Hf(VI) |
H |
-7.107 |
-194.219 |
-15.857 |
-7.714 |
NO2 |
-3.131 |
-201.382 |
-12.520 |
-4.140 |
Cl |
-6.097 |
-195.945 |
-14.260 |
-14.260 |
Table 5. IC50 values of IV1 -
IV15a causing 50 % decrease of oxygen evolution
rate in the algal suspensions of Chlorella vulgaris.
No |
R2 |
R1 |
X- |
log P |
IC50(µmol dm-3) |
IV1 |
CH3 |
Cl |
I |
3.62 |
153 |
IV2 |
C2H5 |
H |
I |
3.44 |
219 |
IV3 |
CH2-C6H5 |
H |
Br |
4.88 |
97 |
IV4 |
CH2-C6H5-4-NO2 |
H |
Cl |
4.83 |
115 |
IV5 |
CH2-CH=CH2 |
Cl |
Br |
4.36 |
138 |
IV6 |
CH2-C[equivalence]CH |
H |
Br |
3.38 |
229 |
IV7 |
CH2-C[equivalence]CH |
CH3 |
Br |
3.84 |
94 |
IV8 |
C8H17 |
H |
I |
5.89 |
36 |
IV9 |
C8H17 |
Cl |
I |
6.41 |
123 |
IV10 |
CH2CH2-C6H5 |
H |
Br |
5.13 |
46 |
IV11 |
CH2CH2-C6H5 |
Br |
Br |
5.92 |
76 |
IV12 |
CH2COOC2H5 |
Cl |
Cl |
3.54 |
195 |
IV13 |
CH2CH2CH2CH2COOCH3 |
Cl |
Br |
4.02 |
124 |
IV14 |
CH2CH2CH2COOC2H5 |
Cl |
Br |
3.97 |
169 |
IV15 |
C2H5COO-CH-COOC2H5 |
Cl |
Br |
3.90 |
119 |
aPhysical
constants and analytical data are published in ref. 4.
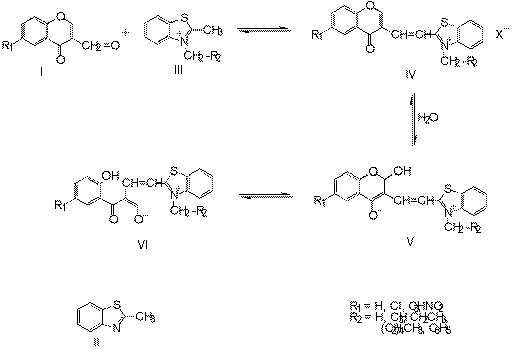
Results and Discussion
We report herein the fast, facile and one - pot - synthesis of
2-[(4H-4-oxobenzopyran-3-yl) ethenyl]benzothiazolium (or bezoxazolium)
salts by condensation of three starting compounds, e.g. 2-methylbenzothiazole
(or 2-methylbenzoxazole), 3-formylchromones and benzylbromide in apropriate
solvents.
We compared the results of classic methods with results using microwave
irradiation. It was found, that N-substituted 2-methylbenzothiazolium salts
were very convenient components for condensations with 3-formylchromones by
both classic and microwaves assisted aldol synthesis. The both used methods
were carried out in five solvents (C2H5OH,
CH3NO2, CHCl3, DMSO, CH3CN) in
order to find favorable reaction conditions for high yields. Using a microwave
oven the reaction time was considerable shorter (5-6 hrs for classical and
8-10 minutes for irradiation conditions). The investigation of the formed
salt in various solvents initiated the study of solvatochromism.
We found that the products from dry nitromethane afforded the higher yields.
The starting compounds as polar molecules absorbed efficiently microwaves and
then reacted very rapidly (8 - 15 minutes) without catalyst and yielded
IV (70 - 90 %).
3-Formylchromones I can be prepared by convenient Vilsmeier double
formylation of appropriate 2-hydroxyacetophenones [17].The preparation of
benzothiazolium or benzoxazolium bromides is published using classic procedures
[4].
We found also that the formation of bezothiazolium bromides III takes
place rapidly under focused microwave irradiation. Creation of compounds
IV by one - pot - synthesis is very effective (method B).
The one - pot - synthesis of benzoxazole analogues of compounds IV was
the most convenient method, the two - step - process (Method A) gave only (20
%) yields. The method for preparation of compounds IV by classic
conditions was generally less effective, yields were about 50 - 60 %.
The optimal structures and quantum chemical parameters (heat of formation and
charge densities) of starting compounds I, III and products
IV, V and VI were calculated by AM1 method with standard
parametrization (keyword PRECISE) [18]. We have found that creation of componds
IV and V are exothermic processes (Table 4.).
Compounds V are more stable than compounds VI (8.1 - 8.3
kcal/mol). Subsituents R1 (H, NO2, Cl) at position 6 of
[gamma]-pyrone ring have a very negligible effect on thermodynamic
characteristics of the reactions and nondecisive influence at reaction sites of
compounds IV (Fig. 1). The distribution of the molecular electrostatic
potential on the van der Waals surface for all compounds IV are very
similar (the most positive values of MEP are in blue area). The reactivity of
all salts IV are also very similar.
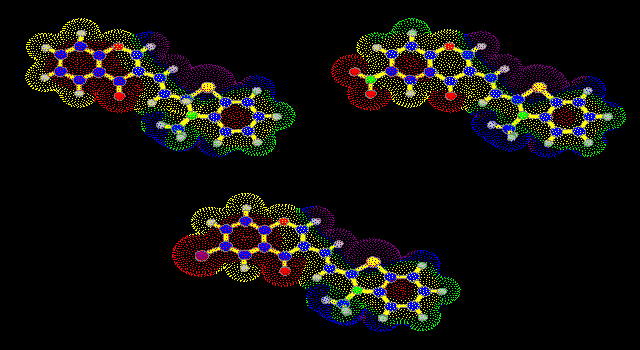
Fig 5. Molecular electrostatic potential on the van der Waals surface
for salts IV (The most positive values are in blue area, R1 =
H, NO2, Cl).
The nucleophilic reactions at C-2 of compounds IV in the presence any
traces of water in solvent led to compounds V or VI. These
reactions were indicated by coloring of samples from yellow to red or
violet.
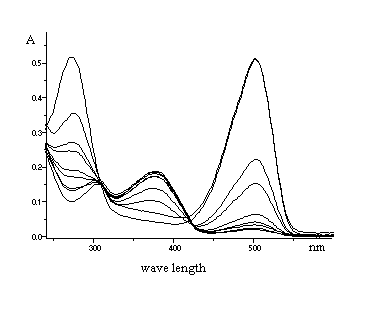
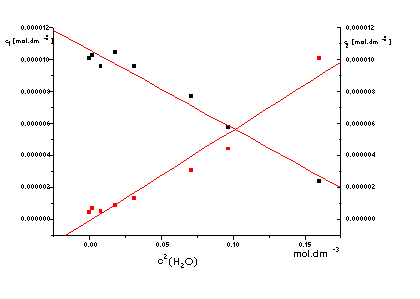
The UV - VIS spectra were used for investigation of interactions compounds
IVa and IVk with solvents (DMF, DMSO, CHCl3) and
especially with water present in solvents (Fig. 2, 3, 4). The absorbance at
1max(378 nm) decreases and at
2max(275 and 502 nm) increases with increasing of water
concentration in methanolic solution of IVa and IVk (Fig. 3, 4).
We found relative small shifts of wavelenght in solvents with different
polarity.
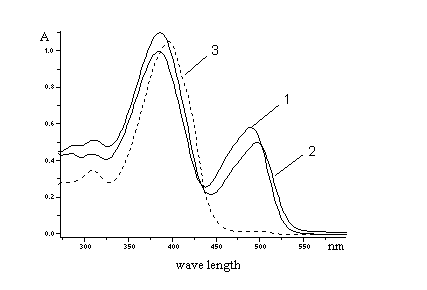
Fig. 2. UV VIS spectra of
3-benzyl-2-[2-(chromon-3-yl)ethenyl]benzothiazólium bromid
(IVa).
Absorbance in various solvents 1. DMF
1max=386 nm, 2max=490
nm
2. DMSO
1max=386 nm, 2max=498 nm
3. CHCl3
max=396 nm