|
|
|
Synthesis of Beta-carboline Derivatives
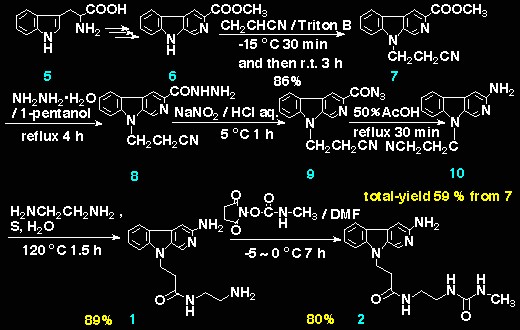
Strategy for the preparation of (1) and (2) is shown in Scheme. Firstly, 3-methoxycarbonyl-beta-carboline (6) was synthsized from L-tryptophan (5) according to a method appeared in literature.3-5 Secondly, Triton B (a 40 % solution in methanol) was added slowly to (6) in acrylonitrile at -15 oC, then, stirred for 30 min at -15 oC and for 3 h at room temperature to give (7, mp 213.0 ~ 213.9 oC) in 86% yield. Next, (7) was applied to Curtius reaction in the following manner 4 : a mixture of (7) and hydrazine hydrate was heated under reflux for 4 h in 1-pentanol to give the hydrazide (8), which was treated with sodium nitrite / hydrochloric acid for 1 h at 5 oC to yield the azide (9). On refluxing for 30 min in 50 % acetic acid, (9) rearranged to 3-amino-9-cyanoethyl-beta-carboline (10, mp 194.4 ~ 195.5 oC) in 59 % total-yield from (7) . In these successive reactions from (7) to (10), crude products of (8) and (9) were used to the next reactions without further purification, owing to their labilities. The compound (10) was heated at 120 oC for 1.5 h in a mixture of ethylendiamine and H2O in the presence of sulfur to give (1, mp > 208 oC (decomp.)) in 89 % yield. Finally, (1) and succinimidyl N-methylcarbamate in dry dimethylformamide gave beta-carboline N-methylurea (2, mp > 210.8 oC (decomp.)) in 80 % yield.