

|

|
The interaction of beta-carboline derivatives (1) ~ (4) with the duplex-DNA oligomer were spectrophotometrically studied . Commercial product of norharman (3) was used without additional purification. 3-Amino-beta-carboline (4) was synthesized from (6) according to a method appeared in literature.5 Optical titrations of beta-carboline derivatives with the duplex-DNA oligomer were shown in Figures 1 a, b. They indicate 25 ~ 65 nm bathochromic shift≠„ of the light absorption bands upon binding to the nucleic acids, which suggests binding by intercalation.7 Scatchard plots8-10 obtained by fluorescence titration (shown in Figure 2) gave the correcponding binding constants KD, which indicates that the aliphatic amino group of (1) considerably decreases the intercalation to DNAs. This suppression of intercalation ability may be owing to the hydrogen-bonding between the aliphatic amino group and an anionic phosphoric acid back-born of the nucleic acids.
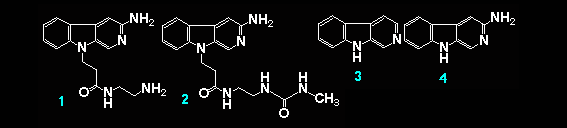
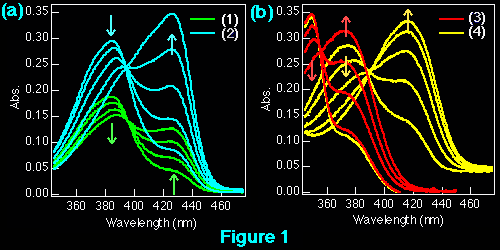
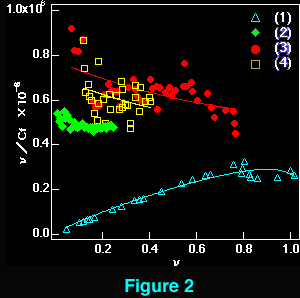
Table. Binding constants, KD, of beta-carbolines (1) ~ (4) with the duplex-DNA, obtained by Scatchard analysis.
Compound |
KD (M-1) |
|
12mer DNA |
Calf Thymus DNA |
|
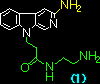
|
7.45 x 103 |
4.61 x 103 |
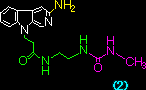
|
5.13 x 105 |
5.91 x 105 |
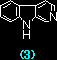
|
7.34 x 105 |
1.63 x 106a) |
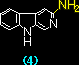
|
7.01 x 105 |
- |


