| [Related articles/posters: 010 087 032 ] |
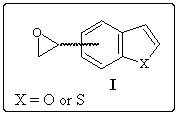 We are going to present the different ways investigated to obtain 4- and 7-oxiranyl-benzo[b]thiophene and furan starting from the corresponding methyl derivatives, which preparations are well known.1, 2
DIFFERENT WAYS OF SYNTHESIS OF 4-AND
7-OXIRANYL-BENZO[b]THIOPHENE AND FURAN
As it can be seen on the following general scheme, starting from the precursor II, three different ways have been investigated.
We are going to present the different ways investigated to obtain 4- and 7-oxiranyl-benzo[b]thiophene and furan starting from the corresponding methyl derivatives, which preparations are well known.1, 2
DIFFERENT WAYS OF SYNTHESIS OF 4-AND
7-OXIRANYL-BENZO[b]THIOPHENE AND FURAN
As it can be seen on the following general scheme, starting from the precursor II, three different ways have been investigated.
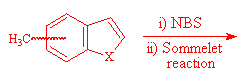
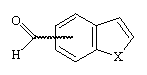

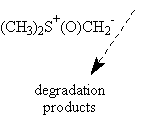
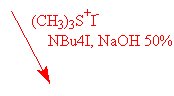
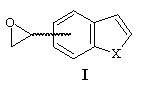
 The formation of 4- and 7-benzo[b]thiophene and furan carboxaldehyde was a key step of the synthesis of 4- and 7-oxiranyl-benzo[b]thiophene and furan I. They have been prepared from 4- and 7- methylbenzo[b]thiophene and furan by bromination using NBS, followed by a Sommelet reaction3.
An obvious way to acceed to epoxydes is the epoxydation of the double bond of the vinyl derivative obtained through a Wittig reaction. This way has been quickly abandonned because it was not possible to perform the Wittig reaction on the aldehydes.
The use of two different oxyranylation reagents from aldehydes has been described in the literature : trimethylsulfoxonium iodide4 and trimethylsulfonium iodide5. We have tried both.
The use of trimethylsulfoxonium iodide under different basic conditions as described did not afford the expected epoxyde I, only degradation products have been observed. On the other hand, trimethylsulfonium iodide under PTC conditions gave the expected oxiranes with yields varying from 60 % to 80% (see table).
Some authors tried to investigate the difference of reactivity between these two similar reagents4 , but there is actually no rule concerning the choice of one or the other of them.
Mostly described in the literature is the use of trimethylsulfoxonium iodide however the difference may be due to the starting material used for preparing these reagents : DMSO in one case, dimethylsulfide (!) in the other.
Table
The formation of 4- and 7-benzo[b]thiophene and furan carboxaldehyde was a key step of the synthesis of 4- and 7-oxiranyl-benzo[b]thiophene and furan I. They have been prepared from 4- and 7- methylbenzo[b]thiophene and furan by bromination using NBS, followed by a Sommelet reaction3.
An obvious way to acceed to epoxydes is the epoxydation of the double bond of the vinyl derivative obtained through a Wittig reaction. This way has been quickly abandonned because it was not possible to perform the Wittig reaction on the aldehydes.
The use of two different oxyranylation reagents from aldehydes has been described in the literature : trimethylsulfoxonium iodide4 and trimethylsulfonium iodide5. We have tried both.
The use of trimethylsulfoxonium iodide under different basic conditions as described did not afford the expected epoxyde I, only degradation products have been observed. On the other hand, trimethylsulfonium iodide under PTC conditions gave the expected oxiranes with yields varying from 60 % to 80% (see table).
Some authors tried to investigate the difference of reactivity between these two similar reagents4 , but there is actually no rule concerning the choice of one or the other of them.
Mostly described in the literature is the use of trimethylsulfoxonium iodide however the difference may be due to the starting material used for preparing these reagents : DMSO in one case, dimethylsulfide (!) in the other.
Table
| starting material | compound obtained | yield % |
| 4-benzo[b]thiophene carboxaldehyde | 4-oxiranyl- benzo[b]thiophene | 77% |
| 7-benzo[b]thiophene carboxaldehyde | 7-oxiranyl- benzo[b]thiophene | 59% |
| 7-benzo[b]furan carboxaldehyde | 7-oxiranyl- benzo[b]furan | 59% |
We are actually studying the reactivity of the oxiranes I towards nucleophiles such as amines to obtain the corresponding
b-amino-alcohols6.REFERENCES
1) T. Yamaguchi, T. Seki and K. Ichimura, Bull. Chem. Soc. Jpn. 1992, 65, 649.
2) T. Q. Minh, L. Chridtians and M.Renson, Tetrahedron. 1972, 28, 5397.
3) N. Blazevic, D. Kolbah, B. Belin, V. Sunjcand and F. Kajfez, Synthesis. 1979, 161.
4) E. J. Corey and M. Chaykovsky, J. Am. Chem. Soc. (C). 1984, 70, 2750.
5) T. S. Kaufman, J. Chem. Soc. Perkin Trans 1. 1996, 2497.
6) S. Chumpradit, H. F. Kung, J. Billings, M. P. Kung and S. Pan, J. Med. Chem. 1989, 32, 1431.