| [Related articles/posters: 029 039 010 ] |
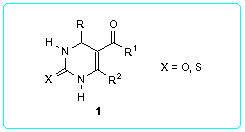
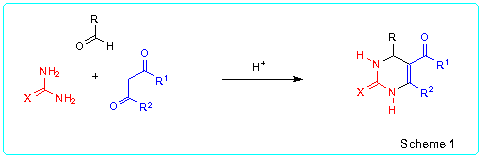
At the present time there are a few general methods of the synthesis of 5-acyl-1,2,3,4-tetrahydropyrimidine-2-thiones/ones. One of them is the Biginelli reaction [2,3] (Scheme 1). This very simple method involves acid-catalyzed three-component condensation of (thio)ureas, aldehydes and b-oxoesters or 1,3-dicarbonyl compounds. The main disadvantage of this synthesis quite often is low yields of the desired pyrimidines because various side reactions occur. For instance, reaction of urea and ethyl acetoacetate with aliphatic aldehydes gives ethyl 4-alkyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylates in yields less than 30-40 % [4].
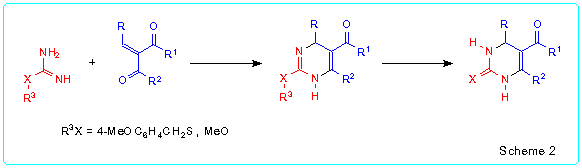
Very attractive approach to the synthesis of Biginelli compounds was developed by Atwal and co-workers [5] (Scheme 2). This approach is based on the reaction of a-arylidene-b-oxoesters with S-(4-methoxybenzyl)isothiourea or O-methylisourea in the presence of sodium bicarbonate followed by transformation of the obtained 2-(4-methoxybenzylthio)- or 2-methoxy-1,4-dihydropyrimidine-5-carboxylates into 2-thioxo- or 2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylates.
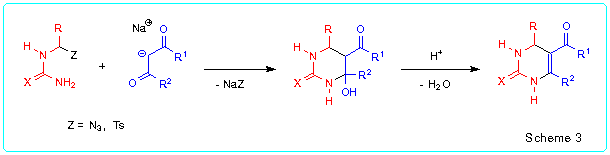
Recently we have demonstrated [6,7] that Biginelli compounds can be easily prepared by reaction of a-azido or a-tosyl substituted thioureas and ureas with sodium enolates of b-oxoesters or 1,3-dicarbonyl compounds followed by acid-catalyzed dehydration of the obtained 5-acyl-4-hydroxyhexahydropyrimidine-2-thiones/ones (Scheme 3). Both the stages of the synthesis proceed under mild conditions and usually in high yields. This method is very flexible and gives possibility to prepare a large number of 1,2,3,4-tetrahydropyrimidine-2-thiones/ones bearing various substituents in pyrimidine ring.
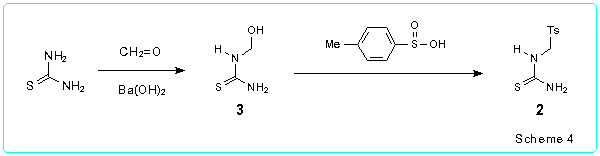
In continuation of our work in this area we developed an improved one-pot procedure for the synthesis of 5-acyl-1,2,3,4-tetrahydropyrimidine-2-thiones/ones starting from a-tosyl substituted thioureas and ureas without isolation of the intermediate 4-hydroxyhexahydropyrimidine-2-thiones/ones. Some preliminary results of the investigation were published in our recent paper [8].
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
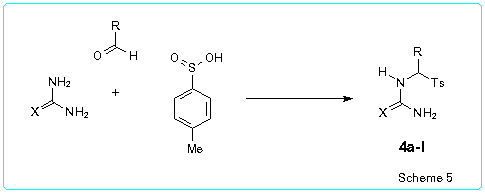
As second building block for the pyrimidine synthesis in the present work we used 1,3-dicarbonyl compounds (acetylacetone 5a, benzoylacetone 5b) or b-oxoesters (ethyl acetoacetate 5c, ethyl benzoylacetate 5d, ethyl butyrylacetate 5e).
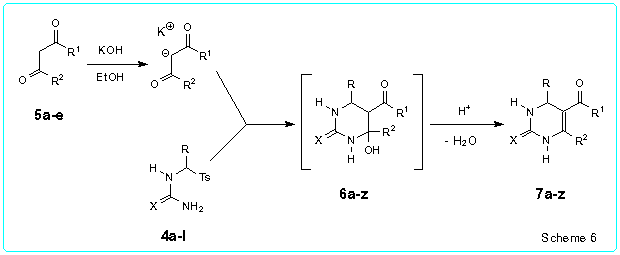
We found that N-(tosylmethyl)thiourea 2 readily reacted with the potassium enolates of the CH acids 5a-d (1.05-1.20 equiv.) generated by treatment of 5a-d with KOH in ethanol to afford the corresponding 5-acyl-4-hydroxyhexahydropyrimidine-2-thiones 6a-d (r.t., 4.5 h). The latter without their isolation were dehydrated after addition of TsOH (0.3-0.4 equiv. for 5a-c and 1.08 equiv. for 5d) to the reaction mixtures and subsequent refluxing for 1-1.5 h. Thus we obtained the 4-unsubstituted 5-acyl-1,2,3,4-tetrahydropyrimidine-2-thiones 7a-d in 71-87 % yields (Table 2) (Scheme 1). It should be noted that workable catalyst of the dehydration of 6a-d is p-toluenesulfinic acid which is generated by reaction of TsOH with sodium p-toluenesulfinate obtained in the first stage of the synthesis.
The described approach was also applied to the one-pot synthesis of the 4-alkyl substituted 1,2,3,4-tetrahydropyrimidine-2-thiones 7e-n and 4-aryl substituted 1,2,3,4-tetrahydropyrimidine-2-thiones 7o-s by reaction of the corresponding thioureas 4a-f with the potassium enolates of 5a-f in ethanol (r.t., 4.5-6 h) followed by acidification and refluxing of the reaction mixtures. The yields of the pyrimidines 7e-s were 46-98 % (Table 2). Similarly, starting from the a-aryl substututed (tosylmethyl)ureas 4h-l we obtained the corresponding 5-acyl-4-aryl-1,2,3,4-tetrahydropyrimidine-2-ones 7t-z in 40-85 % yields.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
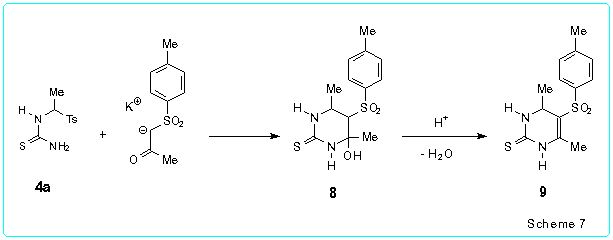
The proposed method for the one-pot pyrimidine synthesis is general and very flexible. This approach was successfully used by us in the cases when the Biginelli reaction and the Atwal procedure failed to give the disired products (see above). Moreover, our method can be applied not only for preparation of Biginelli compounds but also for synthesis of a large variety of hydrogenated pyrimidines. For example, reaction of N-(1-tosylethyl)thiourea 4a with the potassium enolate of tosyl acetone (r.t., 4.5 h) generated by treatment of the CH acid with KOH in ethanol gave the 4-hydroxy-5-tosylhexahydropyrimidine-2-thione 8 which was dehydrated without isolation after addition of TsOH to the reaction mixture and subsequent refluxing for 1 h. Thus we obtained 4,6-dimethyl-5-tosyl-1,2,3,4-tetrahydropyrimidine-2-thione 9 in 55 % yield (Scheme 7).
2. Kappe, C.O. Tetrahedron 1993, 49, 6937-7963.
3. Biginelli, P. Gazz. Chim. Ital. 1893, 23, 360-416.
4. Shutalev, A.D.; Sivova, N.V. Khim. Geterotsicl. Soedin. 1998, in press, and references cited herein.
5. O'Reilly, B.C.; Atwal, K.S. Heterocycles 1987, 26, 1185-1188. Atwal, K.S.; O'Reilly, B.C.; Gougoutas, J.Z.; Malley, M.F. Heterocycles 1987, 26, 1189-1192. Atwal, K.S.; Rovnyak, G.C.; O'Reilly, B.C.; Schwartz, J. J.Org.Chem. 1989, 54, 5898-5907.
6. Shutalev, A.D.; Kuksa, V.A. Chemistry of Heterocyclic compounds 1995, 31, 86-91. Engl. transl. from Khim. Geterotsicl. Soedin. 1995, 97-102.
7. Shutalev, A.D.; Kuksa, V. Khim. Geterotsicl. Soedin. 1997, 105-109.
8. Shutalev, A.D.; Kishko, E.A.; Sivova, N.V.; Kuznetsov, A.Yu. Molecules 1998, 3, 100-106.