| [Related articles/posters: 040 052 104 ] |
Some quinoid heterocycles show biological activity, while containing anthraquinone nucleus condensed with pyrane, azine or azole cycles .
The obtaining of compounds (II), (IV), (V) suitable for obtaining of valuable heterocyclic systems, is reported in this work.
For instance, the esters (II) are obtained by the reaction of esters of 1-nitro-2-anthraquinonecarboxylic acid with NaNO2.
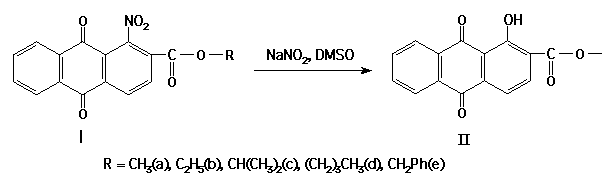
The compounds (IIa-e) have been obtained with high yield and may be applied to obtain analogues of natural anthrapyranes and other oxygen-containing heterocycles of anthraquinone series, taking into consideration the availability of esters (I).
Anthra[1,9-cd;5,10-c'd']bisisoxazole (III) is also of interest. This is stable heteroanalogue of unknown and less thermodynamically stable 1,5-anthraquinone:
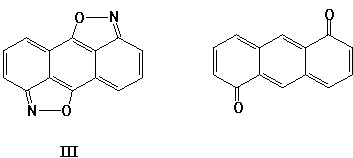
Although there are no carbonyl groups in bisisoxazole (III), all three carbocycles have quinoid (not aromatic) nature, and this fact causes optimism concerning the reactions of compound (III) with nucleophiles.
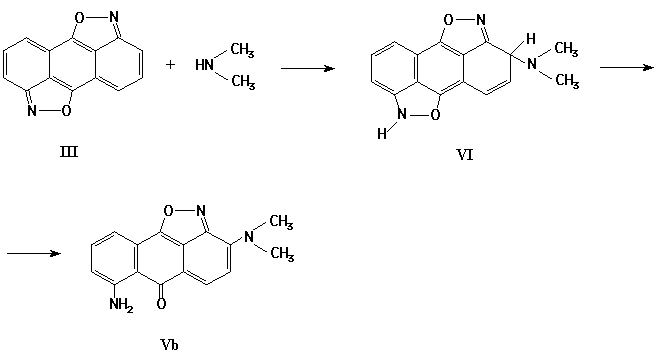
Indeed, the isoxazolone (III) reacts with aliphatic amines at 20-25 žC in DMSO and in DMFA. The best results were achieved in course of amination of bisisoxazole (III) in inert atmosphere.
As a result of interaction of isoxazole (III) with n-butylamine, the mixture of aminoisoxazoles (IV-Va) has been obtained. The reaction of compound (III) with dimethylamine leads mainly to the heterocycle Vb:
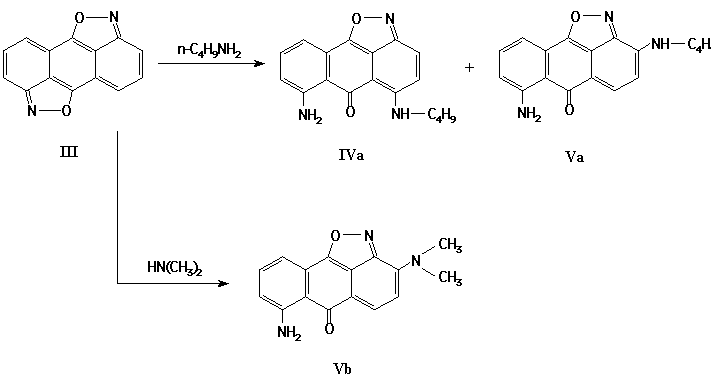
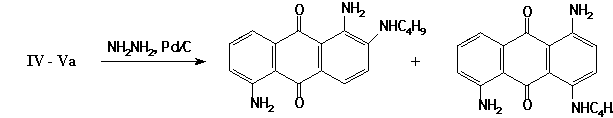
The structures of substances IV-V have been confirmed by spectroscopic data and by their transformation into described [1] 1,5-diamino-2(4)-butylamino-9,10-anthraquinones.
Apparently, the reaction III ® IV-V may be interpreted by analogy with nucleophilic addition of amines to quinones:
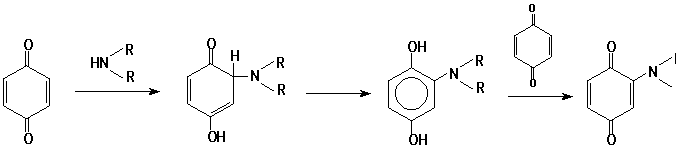
However, further transformations of intermediate (VI) in this reaction are not typical to that for quinones, and include splitting of one of the isoxazole cycles:
Experimental
IR-spectra were recorded on a Specord IR-75 spectrometer for KBr pellets. 1H NMR spectra were measured on a Bruker WP 200 spectrometer with SiMe4 as internal standard and for CDCl3 as a solvent. Melting temperatures were measured on a Boetius melting-point apparatus. The results of the syntheses and purity of the obtained compounds were observed by thin layer chromatography on Silufol plates (eluent: toluene-acetone-hexane; 10:1:1). The data of elemental analysis of the synthesized compounds are quite equal to that calculated.
Syntheses
2-Alkoxycarbonyl-1-hydroxyanthraquinones (IIa-e)
2.5 mmol of initial compound (Ia-e) in 10-15 ml of DMSO (DMFA) was stirred for 7-8 h at 70-75°C. In 15-30 min after beginning of stirring the suspense was already transformed into wine-coloured solution. In 7-8 h after the beginning of stirring the solution was cooled off and the precipitate of the product (IIa-e) was filtered and washed with water and ethanol. The filtrate was diluted by water, the precipitated product (IIa-e) was filtered and washed with water and ethanol.
1-hydroxy-2-methoxycarbonylanthraquinone: mp 158-160°C
2-ethoxycarbonyl-1-hydroxyanthraquinone: mp 128-130°C
1-hydroxy 2-isopropoxycarbonylanthraquinone: mp 112-114°C
2-butoxycarbonyl-1-hydroxyanthraquinone: mp 84-86°C
2-benzoxycarbonyl-1-hydroxyanthraquinone: mp 145-146°C
Anthra[1,9-cd;5,10-c'd']bisisoxazole (III) has been obtained in three steps: 1) diazotization of 1,5-diaminoanthraquinone under the action of nitrosulphonic acid; 2) the reaction between the diazonium salt and sodium azide; 3) the cyclization of obtained 1,5-diazidoanthraquinone by boiling in xylene. Yield 90%. mp > 300°C.
Amination of anthra[1,9-cd;5,10-c'd']bisisoxazole
The suspension of bisisoxazole (III) (25 mmol) in DMAA (20 ml) was saturated with argon; then the 33% water solution of dimethylamine (2 ml) or n-butylamine (2 ml) was added while stirring. The mixture was stirred for 20-80 h at 20-25°C. In course of stirring the suspension was transformed into violet solution.
The isolation of Vb The mixture was cooled off and the precipitate of Vb was filtered and washed with ethanol.
The isolation of IVa and Va The mixture was heated up to 50-60°C, then 5-15 ml of water was added. The mixture was kept at 0 - +5 °C. The suspended product was filtered and purified by column chromatography (adsorbent: Al2O3, eluent: toluene). After the removal of the solvent, the products IVa — Va were analyzed.
5-Butylamino-7-amino-6H-6-oxoanthra[1,9-cd]isoxazole (IVa): Yield 45%, mp 164-166°C.
3-Butylamino-7-amino-6H-6-oxoanthra[1,9-cd]isoxazole (Va): Yield 25%, mp 129-131°C
3-Dimethylamino-7-amino-6H-6-oxoanthra[1,9-cd]isoxazole (Vb): Yield 70%, mp > 300°C
Reduction of IVa, Va
0.1 g of the IVa (Va) in 20 ml of ethanol was reduced by hydrazine hydrate in presence of 0.1 g Pd/C. After the initial products were transformed, the solution was filtered while being hot. The filtrate was diluted with water, and 1,5-diamino-2(4)-butylaminoanthraquinone was isolated.
1,5-diamino-2-butylaminoanthraquinone: Yield 36%, mp 193-195°C
1,5-diamino-4-butylaminoanthraquinone: Yield 46%, mp 178°C
Acknowledgements
This work has been done under the financial support of Krasnoyarsk Regional Science Foundation, grant №: 7F0187
References