| [Molecules: 9] [Related articles/posters: 018 025 016 078 052 ] |
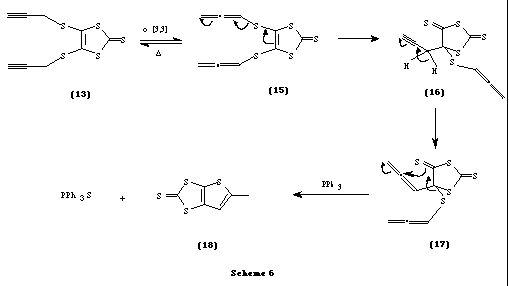
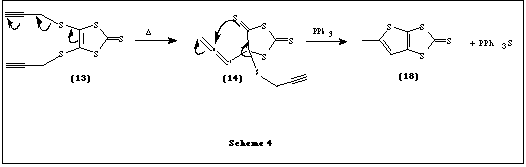
We describe here a convenient route to the synthesis of one such thione. This involves a thio-Claisen rearrangement of 4,5-bis(prop-2-ynylthio)-1,3-dithiole-2-thione (13) to give the novel thione, 5-methylthieno[2,3-d]-1,3-dithiole-2-thione (18) and subsequent coupling of the thione to give [TTF](19) in trace amounts.
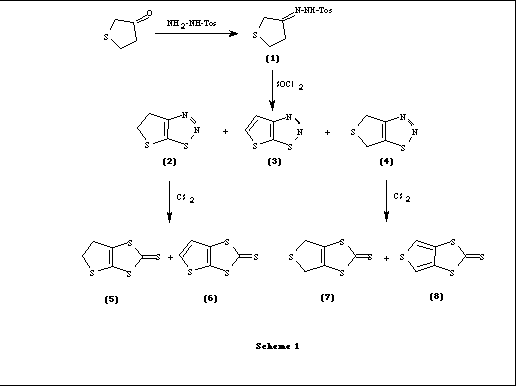
The synthetic routes for these type of donors involve formation of the precursor thiones by thermolysis of the corresponding 1,2,3-thiadiazoles and subsequent trapping of the formed 1,3-dipoles by carbon disulfide. The starting material, tosylhydrazone of tetrahydrothiophen-3-one (1) when treated with thionylchloride gave a mixture of 1,2,3-thiadiazole isomers 2, 3 and 4 [2]. Thermal decomposition of thiadiazoles 2 and 4 in the presence of carbon disulfide resulted in the formation of 1,3,dithiole-2-thiones 5, 6 and 7, 8 respectively (Scheme 1). It is interesting to note that the aromatic thiones 6 and 8 were obtained in very low yields.
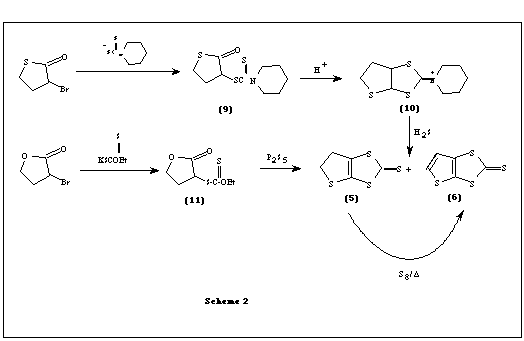
Another method reported [3] for the synthesis of thiophene-fused thiones involves an oxo ester 9 which was cyclized to the iminium salt 10. Treating this iminium salt 10 with hydrogen sulfide gives the precursor thione 5 which can be converted to 6 by sulfur dehydrogenation at 180 oC. The other method reported by the same authors involve an oxoxanthate ester 11 which when cyclized with P4S10 in refluxing decalin afforded the precursor thione 5 as the major product along with smaller amounts of 6 (Scheme2).
We now report a more convenient route to the synthesis of one such thione using a different synthetic methodology.
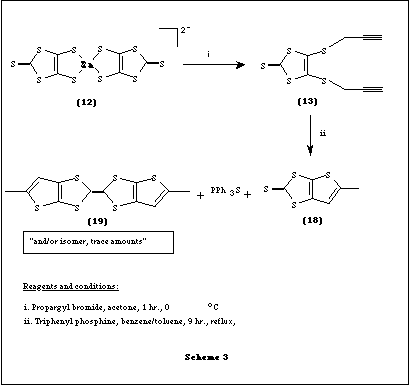
The thione 13 was synthesised by treating prop-2-ynyl bromide with Zn (DMIT)2- salt 12 in acetone at 0 oC for 1 h and eluting the reaction mixture on silica gel using light petroleum/ethyl acetate (95:5) as eluent. However, small amounts of monocyclised product were also obtained.
A probable mechanism for the formation of 18 involving an allenic intermediate 14 is proposed and is shown in Scheme 4. It is interesting to note that thione 18 was not formed on refluxing the thione 13 in benzene-toluene without using triphenyl phosphine, thus confirming the crucial role played by triphenyl phosphine in abstracting the sulfur atom from the intermediate14. The thione 18 then appears to undergo coupling reaction with PPh3 yielding trace amounts of the isomeric mixtures of [TTF] 19 (as identified by mass spectrometry). Recently, some reactions were reported where a single sulfur atom was abstracted from thiones by phosphorous reagents such as P(OEt)3 yielding rearranged thiones or TTFs [5].
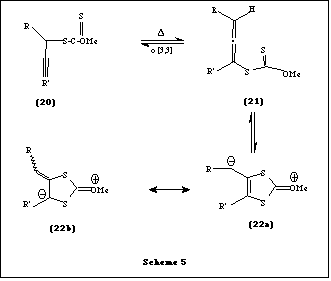
More recently Zard et al. have reported an unexpected, non-radical rearrangement while studying the behaviour of S-prop-2-ynyl xanthates 20 [6]. They found that these substances undergo upon heating a thermal sigmatropic rearrangement to give the corresponding 5-allenyl xanthates 21 which appear to be in equilibrium with a bine 22 (Scheme 5).
In view of this result, we cannot rule out the initial formation of the intermediate 15 which subsequently undergoes thio-Claisen rearrangement and later sulfur abstraction by PPh3 and cyclization to give the thione 18 (Scheme 6).