How general is the imidazolidine fragmentation route to 2-azaallyl
anions?
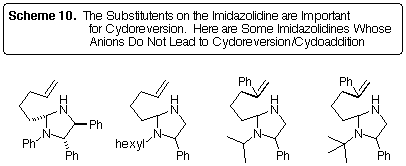
Not very general! Scheme 10 shows some imidazolidines that failed to undergo
cycloreversion/cycloaddition. The first compound shows that an N-aryl
substituent inhibits the reaction. The remaining compounds show that, regardless
of the N-substituent, no cycloreversion is observed where the 2-azaallyl
anion would be unstabilized (i.e., by a C-aryl substituent). Our tin-lithium
exchange methodology is the only method available for the generation of
such unstabilized anions [4].
Conclusion
The cycloreversion of N-lithioimidazolidines to 2-azaallyl anions
has been demonstrated. This method is restricted to the formation of anions
that bear at least one aryl group. An unusual stereochemical complementarity
between the imidazolidine and deprotonation routes to 2-azaallyl anions
was observed. Suggestions on the last point are particularly welcome.
Acknowledgement
We thank the National Institutes of Health forfunding this work.
1. Introduction
2. Observations of Imidazolidine Intermediates in the
Deprotonation Route to 2-Azaallyl Anions
3. Deliberate Generation of Imidazolidines from 2-Azaallyl
Anions, and Their Use as 2-Azaallyl Anion Precursors
4. Synthesis of Imidazolidines from Diamines, and
Their Use in 2-Azaallyl Anion Generation
5. An Unusual Stereochemical Complementarity
6. How General is the Imidazolidine Fragmentation Route to 2-Azaallyl Anions?
and Conclusion (This page)
7. Experimental Section
8. References

