Deliberate generation of imidazolidines from 2-azaallyl anions,
and their use as 2-azaallyl anion precursors
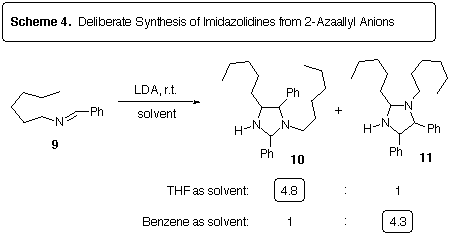
Deprotonation of imine 9, which cannot lead to cycloaddition due
to the absence of an alkene, resulted in the formation of the imidazolidines
10 and 11 (Scheme 4). Interestingly, the regioselectivity
of the dimerization was found to depend on the solvent, providing complementary
results. The stereochemistry of these imidazolidines is unknown, although
the phenyl groups on 11 were later found to be trans by synthesizing
this compound by an unambiguous route.
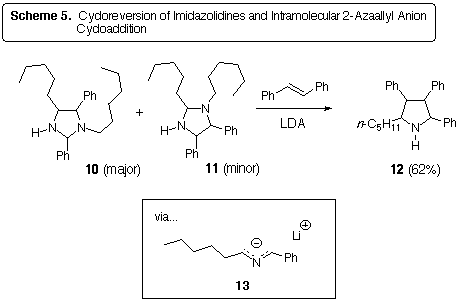
The imidazolidines 10 and 11 were found to be good precursors
of 2-azaallyl anions (Scheme 5). Addition of LDA to a mixture of 10,
11, and trans-stilbene afforded the pyrrolidine 12
as a mixture of three stereoisomers. The imidazolidines used were those
formed in the THF reaction in Scheme 4. Thus, definitive evidence for the
generation of a 2-azaallyl anion (namely, 13) from imidazolidines
has been gathered.
- Introduction
Observations of imidazolidine intermediates in the
deprotonation route to 2-azaallyl anions
-
Deliberate generation of imidazolidines from 2-azaallyl anions, and
their use as 2-azaallyl anion precursors (This page)
-
Synthesis of Imidazolidines from Diamines, and
Their Use in 2-Azaallyl Anion Generation
-
An Unusual Stereochemical Complementarity
-
How general is the imidazolidine fragmentation
route to 2-azaallyl anions? and Conclusion
-
Experimental section
-
References


