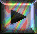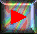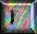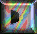Chemical
Synthesis of Melatonin
The methods for the chemical synthesis of melatonin are generally not so complicated and do not involve more than three steps of conversion. Three synthesis reactions of melatonin from primary literatures are shown below;
In 1958 melatonin was first isolated and characterised by A.B.Lerner. It
was know as one of a substituted 5-hydroxyindole derivative in the pineal
gland that could lighten pigment cells. It had not been know to exist in
biological tissue although it had been isolated as a urinary excretion
product in rats after administration of 5-hydroxytryptamine![]() .
.
Melatonin or N-acetyl-5-methoxytryptamine (40 mg) was prepared by reducing
100 mg of 5-methoxyindole-3-acetonitrile with 160 mg of sodium and 2 ml
of ethanol. Then the product was acetylated with 4 ml of both glacial acetic
acid and acetic anhydride at 100 oC for 1 minute. Purification
was achieved by countercerrent distribution and silicic acid chromatography.
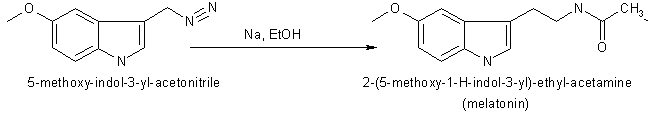
5-Methoxytryptamine hydrochloride (1g, 4.75 mmole) was dissolved in pyridine (10 ml) and acetic anhydride (10 ml) and kept overnight at 20 oC. The solution was poured onto iced, neutralised with dilute hydrochloric acid and extracted with chloroform (2x25 ml). The combined extracts were washed with water, dried in MgSO4 and evaporated to afford a liquid of N,N diacetyltryptamine derivative. The liquid was then poured into water (50 ml) and extracted with chlroform (2x25 ml). The combined organic layers were washed with water (25 ml), dried in MgSO4 and evaporated to dryness. The residual solid crystallised from benzene to afford melatonin 819 mg, 80% yield.
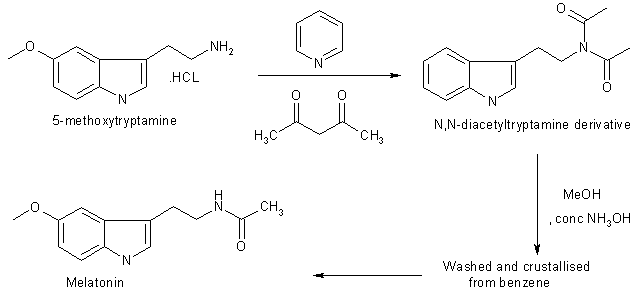
The more reactive indoles (1a-1d) were alkylated at the 3 position by reaction with nitroethene generated in situ by thermolysis of nitroethyl acetate. The nitroethyl acetate used for this purpose was prepared by acetylation of nitroethanol with acetic anhydride using NaOAc as a catalyst. These conditions constitute a substantial improvement of the overal yield of the reation. Reduction of the nitroethylated indoles (2a-d) by hydrogenation over PtO2, followed by acetylation fo the resluting tryptamines with acetic anhydride-pyridine completed the synthesis of melatonin and its derivatives (4a-d).
Biological Synthesis and Metabolism of Melatonin
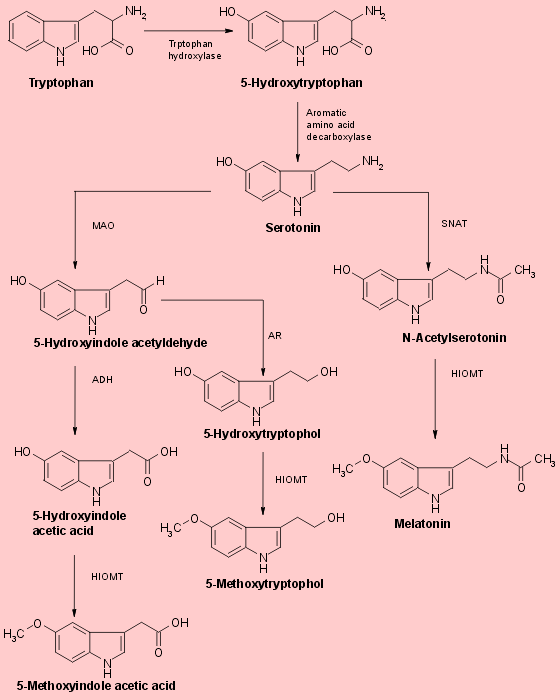
The biosynthesis of melatonin (Fig.1) is initiated by the uptake of the
essential amino acid tryptophan into pineal parenchymal cells. Tryptophan
is the least abundant of essential amino acids in normal diets. It
is converted to another amino acid, 5-hydroxytryptophan, through the action
of the enzyme tryptopahn hydroxylase and then to 5-hydroxytryptamine (serotonin)
by the enzyme aromatic amino acid decarboxylase. Serotonin concentrations
are higher in the pineal than in any other organ or in any brain region.
They exhibit a striking diurnal rhythm remaining at a maximum level during
the daylight hours and falling by more than 80% soon after the onset of
darkness as the serotonin is converted to melatonin, 5-hydroxytryptophol
and other methoxyindoles. Serotonin's conversion to melatonin involves
two enzymes that are characteristic of the pineal : SNAT (serotonin-N-acetyltransferase)
which converts the serotonin to N-acetylserotonin, and HIOMT (hydroxyindole-O-methyltrasferase)
which trasfers a methyl group from S-adenosylmethionine to the 5-hydroxyl
of the N-acetylserotonin. The activities of both enzymes rise soon after
the onset of darkness![]() because of the enhanced release of norepinephrine from sympathetic neurons
terminating on the pineal parenchymal cells.
because of the enhanced release of norepinephrine from sympathetic neurons
terminating on the pineal parenchymal cells.
Another portion of the serotonin liberated from pineal cells after the
onset of darkness is deaminated by the enzyme monoamine oxidase (MAO) and
then either oxidized to form 5-hydroxyindole acetic acid or reduced to
form 5-hydroxytryptophol (Fig.1). Both of these compounds are also
substrates for HIOMT and can thus be converted in the pineal to 5-methoxyindole
acetic acid 5-methoxytryptophol (Fig.1). The level of this latter indole,
like that of melatonin, rises markedly in the pineal with the onset of
darkness. Since 5-methoxytryptophol synthesis does not require the acetylation
of serotonin, the nocturnal increase in pineal SNAT activity cannot be
the trigger that causes pineal methoxyindole levels to rise. More likely,
a single unexplained process- the intraparenchymal release of stored pineal
serotonin, which then becomes accessible to both SNAT and MAO. This process
ultimately controls the rates at which all three major pineal methoxyindoles
are synthesized and generates the nocturnal increases in pineal melatonin
and 5-methoxytryptophol. The proportion of available serotonin acetylated
at any particular time of day or night depends on the relative activities
of pineal SNAT and MAO at that time. The rates of methylation of all three
5-hydroxyindoles formed from pinela serotonin depends on HIOMT activity.