
The chemical shifts and coupling constants of melatonin using CDCl3
as the standard are shown in Table 1 below;
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A variety of methods now exist for the assay of melatonin in body fluids.
Melatonin has the advantage of being non-species specific and hence facilitates
comparison of pineal activity in species with different breeding habits.
Its formation, except in unusual circumstances, appears to be highly localised
in the pineal. Furthermore, as a great deal of information on pineal activity
has been derived from melatonin synthesizing enzymes, it is important to
substantiate this information by measurement of the end product.
Melatonin can be measured by different techniques whose major advantages
and disadvantages are list in Table 1 as shown below;
| Methods | Descriptions |
| Bioassay | Rana pipiens, Xenopus laevis, Nannostomus beckfordi anomalous, specific, intensitive (> 100 pg), tedious |
| Fluorometry | Not specific (lengthy separation procedures), insensitive (>10 ng) |
| Gas chromatography,
mass spectrometry |
Highly specific, Highly
sensitive (<0.01 pg)
|
The quantitative bioassay for melatonin is based on the dermal melanophore
response (nucleocentric aggregation of melatonin granuals) of Larval Rana
pipiens exposed to melatonin in their bathing medium. Groups
of light adapted (melatonin-dispersed) tadpoles are exposed for 30 minutes
to media containing either known concentrations of authetic melatonin or
aqueous extracts of biological samples. They are then killed and fixed
by the addition of formaldehyde to the media. A calebration curve is plotted
relating the melanophore response evaluated microscopically according to
the Hogben index (Waring,1963) to melatonin concentration. From such a
curve the melatonin content of extracts can be accurately estimated.
Melatonin Radioimmunoassays
Various methods have been used to produced anti-melatonin antisera for
radioimmunoassays (RIA) as shown in Table 2. As the molecule is not itself
antigenic, it must be coupled to a carrier molecule. Methods of coupling
melatonin itself or its analoques are shown in Table 2. All these structurally
defferent antigens have produced relatively specific, high titred antisera
of sufficient sensitivity to be used in serum determinations of melatonin.
The type of protein carrier and mode of injection do not appear to influence
greatly the formation of antibodies. Further more, integrity of the N-acetyl
group does not appear to be necessary for specificity. Rabits were used
as experimental animals. Iodinated hapten, iodinated antigen and tritiated
melatonin (3H-MT) have all been used as tracers. A notable advantage
of the iodinated hapten assay system is the high sensitivity of the standard
curve. All RIA methods require prior extraction of melatonin at neutral
of alkaline pH into a non-polar solvent. Further purification by column
or thin-layer chromatography is employed according to the specificity of
the antiserum and the tissue or the fluid to be examined.
| Indol derivative | Protein carrier | Conjugation reation | Hapten/protein molar ratio | Tracer | Titer |
| melatonin | BSA | formaldehyde condensation (Mannich reation) | 3-50 moles/mole | 3H-melatonin |
|
| N-acetyl - 5 - methoxy-tryptophan | TG,BSA | carbodiimide coupling | 10-47 moles/mole | 3H-melatonin | 1:20,000 |
| Indomethacin | HSA | carbodiimide coupling |
|
3H-melatonin | 1:13,000 |
| N-succinyl- 5- methoxy- tryptamine | BSA | mixed anhydride | 30 moles/mole | 125I- N-3- (4
- hydroxy-phenyl)-
propionyl- 5-methoxy- tryptamine |
1:256,000 |
Validation of melatonin RIA
Even after extraction and further prification, the major problem in RIA
of melatonin is that of specificity. Two antibodies, R8 and K244
are used in such system. Classical criteria for the validation of RIA
are shown in Table 3.
| 1. Low cross-reactivity of structural analogues |
| 2. Parallelism between sample aliquots and the standard curve |
| 3. Chromatographic identity of immunoreactivity with melatonin in several solvent systems |
| 4. Good correlation with bioassay and/or gas-chromatogrpahy-mass-spctrometry |
| Further validation of RIA : |
| 1. Consistent high recovery of added melatonin |
| 2. Acceptable intra- and inter-assay variability |
| 3. Low extraction blank |
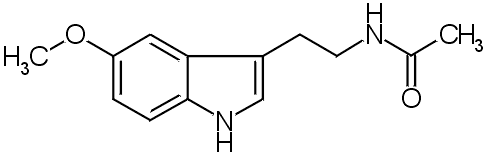
Chromatographic identity of melatonin and selected analoques
The establishment of chromatographic identity of extracted material with
melatonin has been accomplished using primarily the four solvent systems
shown in Fig. 1. The Rf values of selected melatonin analoques are shown
in order to illustrate the usefulness of the silica gel G-butanol system,
and the difficulty of separating N-acetyltrptamine from melatonin.
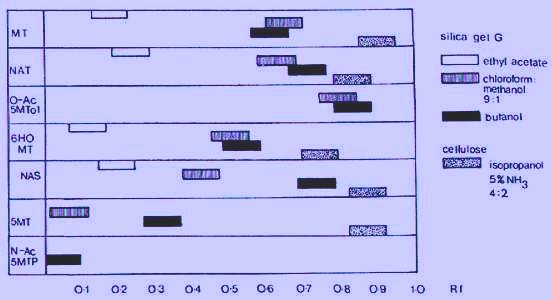
Using K244 antibody, immunoreactive materials other than melatonin were not found. R8 antibody shows a little interference by two other unidentify products as well as possibly N-acetyltryptamine, as shown in Fig. 2. Melatonin in urine poses considerable problems compared with other fluids. Extracted urine in the K244 system shows several immunoreactive peaks on chromatography. It is possible to measure melatonin in urine with a reasonable degree of specificity by RIA after prior chromatographic separation but the procedure is tedious. High pressure liquid chromatography (HPLC) can be used to improve the tecnique.
GCMS is probably one of the most effective methods in the detection and
identification of unknown compounds of for the assay of compounds which
are difficult or impossible to estimate by other methods.
It is a tecnique when the abundance
of one or more ions is monitored continuously as the compounds elute from
the gas chromatograph into the mass spectrometer ion source. A concurrent
increase of two or more ions at the proper retention time and in the proper
ion ratioo can be taken as a strong evidence for the pressence of a given
compound. In the study of pineal chemistry, mass spectrometer was applied
as early as 1959 for the identifecation of 5-hydroxyindole acetic acid
which had been isolated from bovine pineal (Lerner et al., 1958).
Basic function of MS
In the ion source of the mass spectrometry, sample material is ionized, conventionally by an electron beam. The positively charged ions thus formed are accelerated through a potential field, after which they enter the mass analyser, which can either be a magnetic sector of quadrupole field. In either case, the fragment ions are separated according to their mass-to-charge ratio (m/e) and detected by a secondary electron multiplier. Since the charge on an ion in this case is normally equal to one, the m/e usually corresponds to the mass of the ion in atomic mass units (amu). Scanning the field produced a mass spectrum which in almost all cases is unique for a given compound.
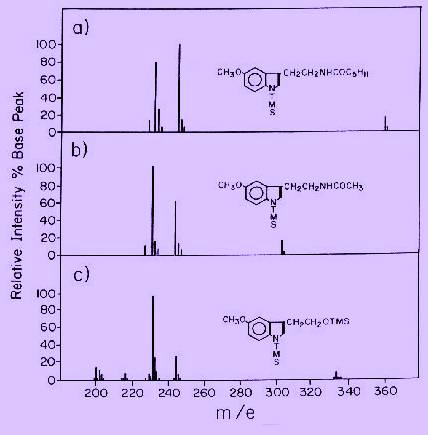
GCMS assay for melatonin
The melatonin assay in this case is based upon selected ion monitoring of the m/e 232 and /or 245 ions from trimethylsilyl (TMS) derivatives (Fig. 3). An internal standard is simply an analoque of melatonin having a considerably longer retention time, with intensed ions at m/e 232 and m/e 245. These ions, which have exactly the same structure as those arising from melatonin, can be used as standards for high resolution measurements on melatonin as well as other methoxy indoles. This type of standard is preferred to deuterium labeled analoques because of problems with deuterium exchange, and difficulty in preparing such compounds with high isotopic purity.