Fig. 2 3D structure of FRAP/FKBP/rapamycin complex
Structure of the FKBP-rapamycin complex
Binding to FKBP
To achieve inhibition of the IL-2 signal to the T cell, rapamycin must first bind to FKBP-12, the same protein that FK506 binds to. This complex has been shown to be neccassary, but not sufficient for inhibition. The binding of rapamycin inhibits the rotamase activity of FKBP, but it is not thought that this leads to its immunosuppressive properties. It is believed that the complex binds to and inhibits FKBP Rapamycin Associated Protein (FRAP) and that it is this association which leads to the inhibition of T cells. Rapamycin fits neatly between the two proteins, but will only combine with FRAP once first complexed to FKBP. Rapamycin binds to the hydrophobic pockets of the two molecules and thus holds them together.
Fig. 1
In the figure shown above the protein coloured blue is FKBP and the one coloured green is FRAP, while rapamyin sits in the centre.
The diagram below shows more effectively the 3-dimensional surfaces of the proteins (the red protein is FRAP and the blue one FKBP):
Fig. 2 3D structure of FRAP/FKBP/rapamycin complex
Structure of the FKBP-rapamycin complex
FKBP (FK506 Binding Protein) is a protein made up of 107 amino acids which catalyses the cis-trans isomerisation of peptidyl-prolyl amide bonds in peptide substrates. The sites of hydrogen bonding which bind the protein to rapamycin have been studied11, 12.
The FKBP protein forms a five-stranded antiparallel beta-sheet wrapping around a short alpha-helix. Rapamycin binds in a cavity between the beta-sheet and alpha-helix with the pipecolinyl ring deeply buried in the protein. The ligand is bound through the region from the pyranose ring to carbon 28, with the remainder, including the triene region, exposed. In the diagram below the binding domain of rapamycin is shown in blue.
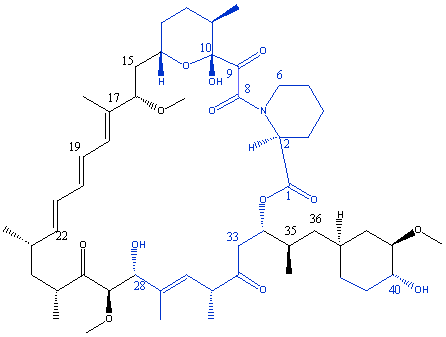
The remainder of the molecule, left exposed after binding to FKBP, is free to bind to FRAP. It is believed that the most interactions occur between FRAP and the triene region of rapamycin.
The table below summarises the hydrogen bonded interactions between rapamycin and the amino acid residues of FKBP.
|
Group on rapamycin |
Amino acid residue of FKBP-12 |
|
C1 carbonyl |
Ile-56 NH |
|
C8 carbonyl |
Tyr-82 OH |
|
C10 hydroxyl |
Asp-37 carboxylate |
|
C9 carbonyl |
Tyr-26, Phe-36 and Phe-99 H |
|
C28 hydoxyl |
Glu-54 carbonyl |
|
C40 hydroxyl |
Gln-53 carbonyl |
Fig. 1 from Brookhaven Protein Databank
Fig. 2 from: http://www-schreiber.chem.harvard.edu/home/structure.html