 |
 |
 |
 |
 |
 |
 |
 |
 |
| |
| Any
suggestions or comments, please contact me at |
| shuyi.quek@ic.ac.uk |
| |
| |
| |
| |
| |
|
- Synthesis -
With
its complex structure and various functional groups, Tetrodotoxin (TTX)
has been a
formidable target for total synthesis
- The structure was first elucidated by Woodward in 1964 and was later
confirmed by Kishi in 1965
-
In September 1972, Kishi and co-workers (Kishi, Y. et al. J. Am.
Chem. Soc. 1972, 94, 9217-9221)
successfully synthesized a racemic mixture of the Tetrodotoxin molecule
-
The reaction is complicated involving a total of 29 steps, including
a ketalization, a Meerwein-Ponndorf-Verley reduction, selenium
oxidation, epoxidation, a Diels-Alder cycloaddition
amongst other techniques.
The
diagram below summarizes the main steps.
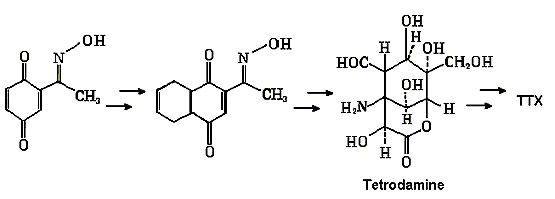
"Kishi synthetic pathway for TTX"
- In January
2003, Isobe and his co-workers (Isobe, M. et al. J. Am. Chem. Soc.
2003, 125) from
Nagoya University, Japan, have
accomplished the first asymmetric total synthesis of Tetrodotoxin.
- This complex reaction involves a series 67 steps. The highlights of
the Isobe reaction are:
~ Chiral starting material of
2-acetoxy-tri-O-acetyl-D-glucal
~ Formation of hydroxylated
cyclohexane core ring
- Claisen rearrangement
- Regioselective hydroxylation of acetone moiety
- Sonogashira coupling (Sonogashira, K. et al. Tetrahedron Lett.
1975, 16, 4467-4470)
synthesizes the precursor required for aldol condensation
- Intramolecular directed aldol condensation
~Introducing
the nitrogen functionality
- Initial attempt using Overman rearrangement (Overman, L.E. Acc.
Chem. Res. 1980, 13,
218-224) was
unsuccessful
- New strategy of intramolecular carbamate-ester (unsaturated)
conjugate addition was employed
The
synthesis of Tetrodotoxin in an enantiomerically pure form was possible
because:
1) Different protective groups were chosen for each íVOH group, this
also to discriminate for
future analog synthesis
2) All stereogenic centers were controlled with high selectivity
Finally,
TTX was purified by ion exchange chromatography and found to be
identical to the
natural molecule.
Back to
Top
|