 |
 |
 |
 |
 |
 |
 |
 |
|||||||||||||||||||||||||||||||||||||
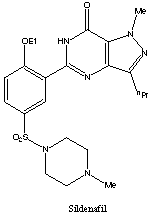
| The chemical name of sildenafil is 5-[2-ethoxy-5-(4-methylpiperazin-1-ylsulfonyl)phenyl]-1- methyl-3-propyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one and its formula is C22H30N6O4S. The melting point of sildenafil is 189-190oC. Its solubility is 3.5 mg/mL in water. |
|||||||||||||||||||||||||||||||||||||
 |
|||||||||||||||||||||||||||||||||||||
The 1H NMR data of sildenafil is given below. The abbreviations used are s for singlet,
d for doublet, t for triplet and q for quartet. The chemical shifts are given in ppm (parts per million) and are
followed by the number of Hydrogens the peaks account for:
|
|||||||||||||||||||||||||||||||||||||
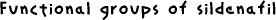
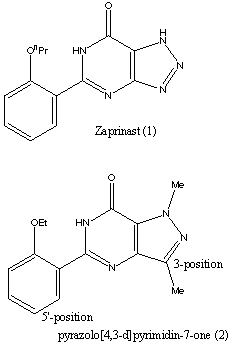
Viagra aims at inhibiting the enzyme phosphodiesterase PDE5. It must therefore have a structure that is similar in some places to the substrate. However, there are many other constraints as there are several different types of PDE enzymes which are found in different parts of the body. Of the 7 types of PDE, three selectively hydrolyse cGMP relative to cAMP. PDE5 itself can be found in several parts of the body : the lungs, platelets and various forms of smooth muscle. Selectivety was a very important factor in the research for an inhibitor of PDE5. The research on a molecule to inhibit PDE type 5 started with the molecule Zaprinast (1) which is shown on the right. The research established that derivatives of pyrazolo[4,3-d]pyrimidin-7-one (2) gave more potent cGMP PDE5 inhibition. The studies carried out compared many molecules by changing the substituents on them and comparing the inhibitory data of these molecules. To establish the selectivity of the compound, the compounds were also screened against the other widespread cGMP enzymes, PDE1 which was isolated from rat liver and PDE3 which was isolated from rat platelet. Further studies showed that sildenafil was the best PDE5 inhibitor. The different functional groups on the molecule were established by comparing the affinities of the molecules to the different PDE enzymes and establishing the selectivity of each compound made. Of course, high selectivity for PDE type 5 was looked for. Viagra mimics the guanosine base of cGMP and the extension of 3-substituent fills a space in the enzyme active site occupied by ribose. Substituents on the 5'-position of the phenyl ring reproduce the role of the phosphate bonding. To improve the solubility of the drug, polar substituents were added which gave compounds with a lower lipophilicity. This was found to also increase the enzyme affinity. Sidenafil gave an excellent combination of enzyme inhibitory potency, selectivity, solubility and in vivo characteristics. |
|
||||||||||||||||||||||||||||||||||||
It is interesting to compare the structure of sidenafil with that of cGMP (cyclic guanosine monophosphate), which is the molecule that usually interacts with PDE5. The drug that was being developed by the researchers had to have some similarity so that it could bind to the active site of the enzyme, PDE5. The 2 dimensional structure of cGMP is given above the 3 dimensional picture, however the latter can be used to view the molecule in 2D as well, with any orientation of the molecule. The first figure also shows that cGMP and pyrazolo[4,3-d]pyrimidin-7-one, which is one of the parent molecules of sildenafil, have a similar size, shape and dipole moment. These were characteristics that were looked for in a molecule that would inhibit PDE5. The following molecule is cGMP :  |
|||||||||||||||||||||||||||||||||||||
|
Cyclic Guanosine Monophosphate (cGMP): |
|||||||||||||||||||||||||||||||||||||