Light and Colour
Fireworks have lit up the night sky for centuries however, colour is an invention that has only been introduced into displays in the last 100 years.
Before the 19th Century, the only colours that could be produced were yellows and oranges with the use of steel and charcoal. Later development involved Chlorates which introduced basic reds and greens to the repertoire. Good blues and purples were not developed until this century and the quest for the formation of a deep forest green coloured firework continues still to this day.
Colour production in fireworks involves two main mechanisms:
1) Incandesence
2) Luminescence
Incandescence is the light produced from heat. Heat causes a substance to grow hot and glow. This results in the emission of infrared, then red, orange, yellow, and white light as the substance grows increasingly hotter. The power of this emission can be defined as:
M = sT4
Where s is the Stefan-Boltzmann constant and T is the temperature.
It is apparent from the equation that a twofold increase in emission can be caused by increasing the flame temperature from 400K to 800K.
The calculated emission spectrum has the following shape:
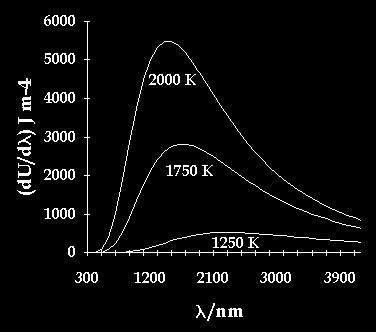
The graph illustrates that different temperatures produces a different wavenumber with a different power of emission and so produce a different colour of light.
The following table gives a summary of the temperatures at which various colours will occur.
| Temperature (K) | Temperature (0C) | Subjective Colour |
|---|---|---|
| 750 | 480 | Faint red glow |
| 850 | 580 | Dark red |
| 1000 | 730 | Bright red/orange |
| 1200 | 930 | Bright orange |
| 1400 | 1100 | Pale orange/yellow |
| 1600 | 1300 | Yellow/white |
| >1700 | >1400 | White |
Blues and greens require much higher temperatures (ones which are impractical for fireworks) and so cannot be formed using this method.
Instead, they are created via the mechanism of Luminescence.
Luminescence is light produced using energy sources other than heat. This involves the absorption of energy by an electron of an atom or molecule thereby causing it to become excited but also unstable. As the electron drops back down to its ground state, energy is released and in this case, it is in the form of light as the energy is released in the form of a phonon. The energy of the phonon determines its wavelength and colour.
Certain molecules emit light in the visible region and these are used to produce the colours seen in fireworks. Some of these however need to be combined with another element to stabi lise them and make them fit for use within fireworks. For example, Barium must be combined with chlorinated rubber as it is unstable at room temperature. On the contrary, Copper Chloride is instable at high temperatures and it must be ensured that the firework does not get too hot.
The following table illustrates the compounds required to produce specific coloured fireworks.
| Colour | Compound | Wavelength of Light |
|---|---|---|
| Red | Strontium Salts & Lithium Salts Li2CO3 SrCO3 | 600-646nm |
| Orange | Calcium Salts CaCl2 CaSO4.2H2O | 591-603nm |
| Gold | Incandescence of Iron or Charcoal | 590nm |
| Yellow | Sodium Compounds NaNO3 Na3AlF6 | 589nm |
| Electric White | White Hot Metal BaO | 564-576nm |
| Green | Barium compounds with Chlorine BaCl+ | 511-533nm |
| Blue | Copper Compounds and Chlorine Cu3As2O3Cu(C2H3O2)2 | 460-530nm |
| Purple | Mixture of Strontium (red) and Copper (blue) compounds | 432-456nm |
| Silver | Burning aluminium, titanium or magnesium powder. | 412nm |
In order to choose the colours of fireworks which are most pleasing to the eye, pyrotechnicians use a Chromacity Diagram as illustrated below:
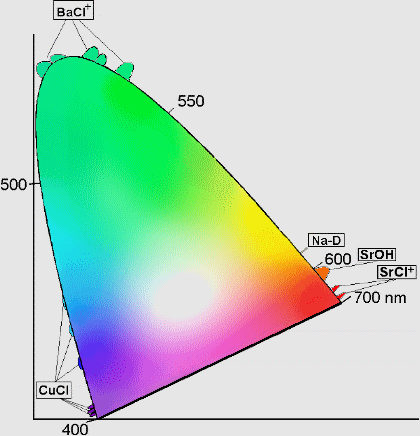
This is designed using the three primary colours red, yellow and blue. In theory, if fireworks of these colours can be produced then the production of a firework of any colour is possible.
The wavelength at which every colour lies can be found on the curved line surrounding the tongue shaped region of the composite colours. The numbers along the curve represent corresponding wavelengths in nanometres.
Unfortunately, the case is not as simple as selecting a colour and finding the corresponding wavelength. Some emitting molecules are so reactive that they cannot be directly packed into a firework. Therefore, the relevant molecules must be evaporated into the flame to keep them at as high a temperature as possible.
To achieve maximum light output substantial amounts of emitters must be present in the flame alongside 5 other vital ingredients.
It has taken pyrotechnicians years to solve the problems which lie behind coloured flame production. Success has been attained in all areas save for the production of the much sought after ocean and forest green coloured firework.
A combination of green and blue coloured emmiters (such as BaCl and CuCl) is the obvious choice however BaCl emission is very rare as they occur as BaOH and BaO emissions which fall in the yellow-green section of the spectrum. Hence the quest for the forest and ocean green coloured fireworks still continues.