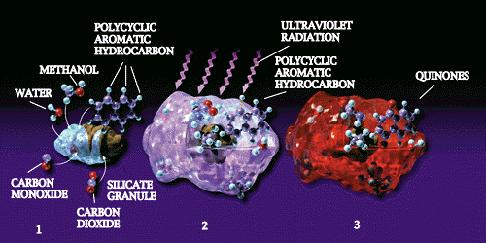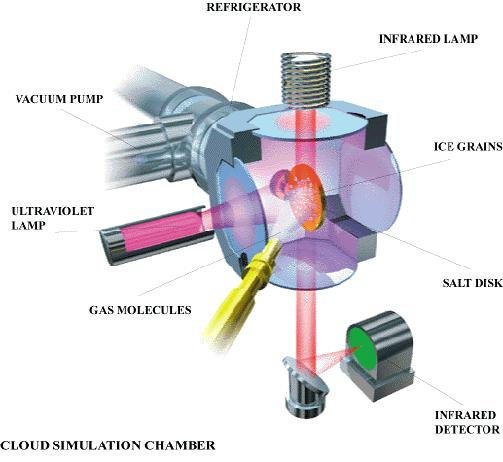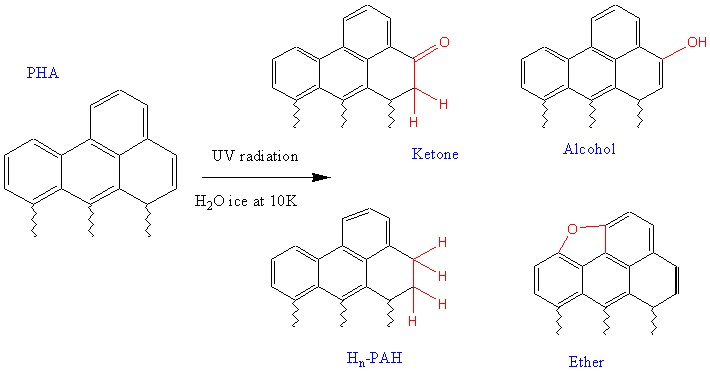
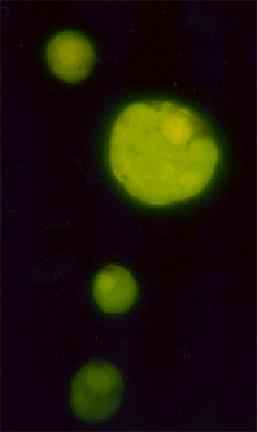
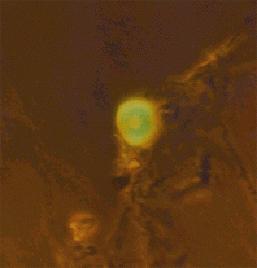
Fig.9(4) and Fig.10(4)
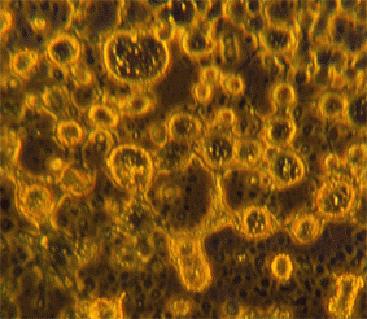
The primitive cells are shown in the photographs fig.8-10. The cells are fluorescing green due to a dye being captured inside their structure like air in a bubble. The trapping of this dye is evidence for the space inside the cell being cut off from the outside environment. The cells are formed by molecules arranging themselves into a membrane with hydrophobic, water hating, parts of the molecules hiding from the water in the inside surface of the bubble.
| Start | Contents | Abstract | Introduction | Sugar in space |
| Spectroscopy | Polyatomic Aromatic Hydrocarbon | Simulating Space | Conclusion |
| References |