|
|
Synthesis
Racemic synthesis
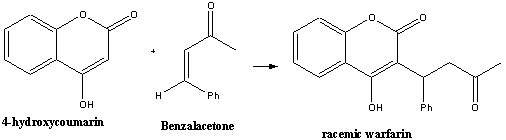
A classic way of synthesising racemic warfarin is by the base
(or acid) catalysed Michael condensation reaction of 4-hydroxycoumarin
with benzalacetone either in water or piperidine2,3.
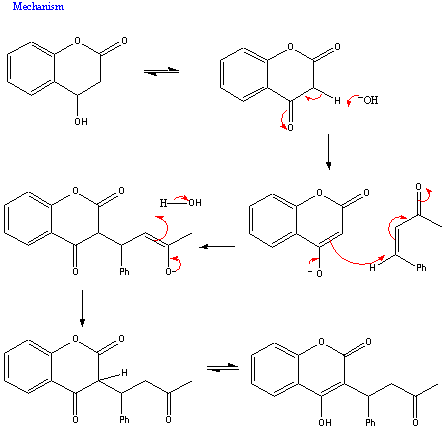
The mechanism for this reaction is as follows:
The yield for this synthesis when carried out in water2
(4-8 h reflux) is typically 40 %. Higher yields3
can be obtained by carrying out the reaction in methanol (20 h reflux),
isolating the product formed and hydrolysing with acid. Typical yields
are 93 %.
Asymmetric synthesis
There has been increasing interest in the pharmaceutical industry
to replace existing racemic drugs by their pure enantiomeric form
due to the fact that one enantiomer often has a pharmacological profile
superior to the racemate. In this case, S-warfarin is found to be 5
times more potent as an anticoagulant than R-warfarin. Preparation of
enantiomerically pure warfarin can be made by the classical resolution
of the racemate using quinidine/quinine salts4
or chromatographic separation. However, these methods are limited to
small scale preparations.
1. Asymmetric hydrogenation
In 1996, researchers at the Dupont Merck pharmaceutical company
developed a practical asymmetric synthesis of R- and S- warfarin starting
from the racemate and using DuPHOS-Rh(I) catalysed hydrogenation.5
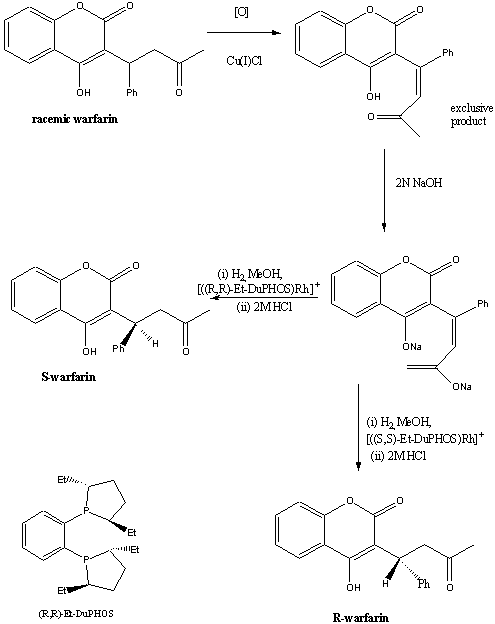
Using this method, S-warfarin was obtained in 83% enantiomeric
excess (e.e) , and R-warfarin in 86% e.e
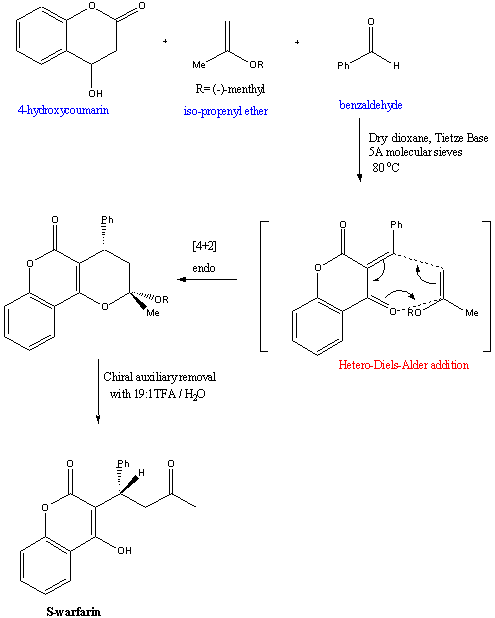
2. Hetero-Diels-Alder cycloaddition
The previous synthesis used racemic warfarin as its starting
material. A novel approach to asymmetric synthesis of warfarin using
simpler starting materials was developed in 2001. It proceeds by a
hetero-Diels-Alder cycloaddition.6
In this reaction, S-warfarin was obtained in 95
% e.e
|