

|
|
|---|
![]()
Historical![]()
Zingerone has been extracted from ginger for the past two thousand years.
A Brief History
| Common Name: |
Ginger |
||||||||
| Latin Name: | Zingiber Officinale | ||||||||
| Family: | zingiberaceae | ||||||||
| Other Names: | Based on its
origin:
|
The word ginger comes from the ancient Sanskrit 'singabera', meaning 'shaped like a horn'. It first appeared in the writings of Confucius in the 5th century BC. and it has been used medicinally in the West for the past 2000 years. Various virtues have been ascribed to the spice; e.g. Henry VIII recommended it as a pro-phylactic against the plague. It was introduced by the Spaniards to the Americas and is now cultivated extensively in the West Indies. The Portuguese introduced it to West Africa. It is now used all over the world.
Only in the past century has zingerone been produced synthetically. A few interesting and unique syntheses have been chosen. Down the years, the technique of synthesis evolved. Though Lapworth & Wykes were the pioneers in the synthesis of zingerone, their method and reagents were not repeated. Below is a diagram of the apparatus used in their experiment.
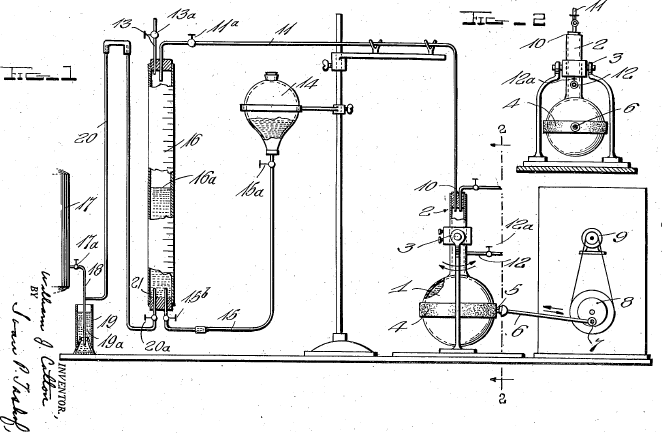
Nomura, then Mannich and Merz founded the method used today. This is shown by Kim and Kim's work. The other interesting method was formed by Bunce and Reeves. This method and the common one used today are elaborated on in the preparation section.
|
Year |
Method | Reagents |
| 1917 |
Reduction
and Decarboxylation of ethyl vanillylideneacetoacetate Lapworth & Wykes |
The Pioneers in the synthesis. Na Amalgam and NaOH solution
|
| 1925 |
Reduction of
4-(4-hydroxy-3-methoxyphenyl)buten-2-one to
4-(4-hydroxy-3-methoxyphenyl)butan-2-one. Nomura |
Na Amalgam and Water
|
| 1927 |
Reduction of 4-(4-hydroxy-3-methoxyphenyl)buten-2-one to 4-(4-hydroxy-3-methoxyphenyl)butan-2-one. Mannich & Merz |
Pd Catalyst,
|
| 1979 |
Reaction of 4-benzyloxy-3-methoxybromomethylbenzene with the anion of acetone dimethylhydrazone, followed by oxidative hydrolysis, then hydrogenolysis to remove the benzyl group
Enders D. et Al |
Pd/C, Hydrogen, Methanol
|
| 1989 |
Amberlyst-15-catalyzed addition of 3-buten-2-one to the phenol Bunce & Reeves |
Amberlyst-15, Toluene, 20-50°C
|
| 2004 |
Reduction of 4-(4-hydroxy-3-methoxyphenyl)buten-2-one to 4-(4-hydroxy-3-methoxyphenyl)butan-2-one. Kim D. & Kim J. Y. |
Pd/C Catalyst,
|