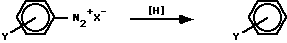
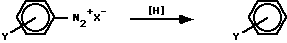
Although two dozen methods exist for effecting this transformation, there are only four reports in the literature of uncatalyzed hydrogen transfer from solvent alone: (a) 2-phenyl-1,3-dioxolane (PDO) [1] , (b) tetramethylurea (TMU) [2] , hexamethylphosphoramide (HMPA) [3] , and dimethylformamide (DMF) [4] . The intermediate species in these systems was considered to be a phenyl cation in PDO and TMU and a phenyl radical in HMPA and DMF. Of these four solvents, only DMF provided two different sites for H abstraction. Recent work by us and others [4] has established that in DMF the pathway is a radical process and that the H-atom source can be either the methyl group or the formyl group.
In order to understand this process better, we have carried out protodediazoniation using DMF, DMF-d1, DMF-d6 and DMF-d7 and measured the nitrobenzene/nitrobenzene-4d1 ratios as a window on the site-selectivity of the hydrogen atom abstraction.
We have also carried out ab initio calculations of the energies, geometries and vibrational frequencies of important species in this reaction. Because of its size, DMF is a challenging problem for high-level ab initio calculations; however, it is tractable using the Gaussian 2 methodology.