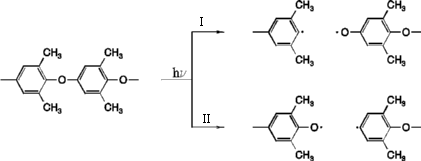
Scheme 1: Mechanism of ether bond cleavage

Scheme 1: Mechanism of ether bond cleavage
Based on there experimental studies Rivaton and Morel [1] suggested that the primary step is a photoinduced ether bond cleavage. This can occur on two different positions, but leads in both cases to a phenyl and a phenoxy radical. The results for this mechanism are summarized here.
Although for both pathways the activation energy is lowered from approximately 230 kJ/mol in the S0 to around 90 kJ/mol in the S1 state, this is still too large for the ether bond cleavage to be reasonable. This is different for the triplet state. Here the activation energy for ether bond cleavage I is 39 kJ/mol. For the second pathway no activation barrier was found. Therefore ether bond cleavage is possible along the second pathway in the triplet state.
The different behaviour between the reaction pathways I and II can be understood if one considers the difference of the final products. The calculated energy difference of the phenyl radicals of 13 kJ/mol can be attributed to a large amount to the energy necessary to rotate the terminal OH-group from the planar to the perpendicular conformation (10 kJ/mol). In contrast, the difference in the heat of formation of the phenoxyradicals is 22 kJ/mol. This indicates that the para-OH-group, which is in-plane for the second pathway, considerably stabilizes the phenoxyradical.
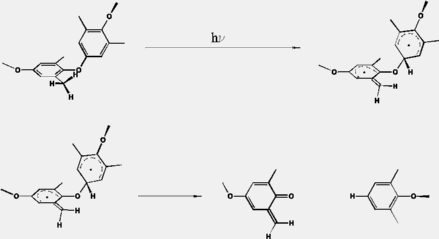
Scheme 2: Mechanism of 1,5-Hydrogen transfer
The results for this mechanism are summarized here.
Similar as for the photoinduced ether bond cleavage the activation energy for the 1,5-hydrogen transfer is reduced from 190 kJ/mol in the ground state to 60 kJ/mol in the S1 state. This barrier is too large, so that the transfer is improbable in the excited singlet state. But again, after intersystem crossing to the triplet state, the transfer can occur without a barrier.
The hydrogen transfer is not only favoured by energetical reasons, but also the steric arrangement of the ground state geometry of the polymer is favourable for the hydrogen transfer. This can be seen here. The distance of the hydrogen to the ipso-carbon atom is 2.7 A and the C-H..C bond angle is 118o. This is acceptable for hydrogen transfer.
After hydrogen transfer, it is reasonable to assume that the formed biradical undergoes intersystem crossing to the S0-ground state. In this state, the system can react in two different ways. The hydrogen can be back transferred to the educt or the ether bond can break. For both reactions an activation barrier of around 55 kJ/mol was calculated. This suggests that both pathways are equally probable. But if one has a closer look at the minimum structure of the biradical the back transfer seems to be rather unlikely. On the one hand, the transferred hydrogen points away from the methylene group, leading to an C-H..C bond angle of 70O. On the other hand, the formed methylene group is now planar and its conformation is unfavourable for the back transfer. Thus the ether bond cleavage should be the favoured pathway.
 Contents
Contents
 Computational Method -
Conclusions
Computational Method -
Conclusions

 Results
ether cleavage -
Results 1,5-hydrogen transfer
Results
ether cleavage -
Results 1,5-hydrogen transfer