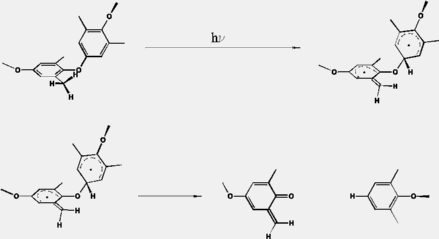

In contrast to the ether bond cleavage, this mechanisms consists of two subsequent steps. In the first step, a hydrogen is transferred from a methyl group to the ipso carbon of the adjacent monomeric unit. The formed biradical can than dissociate in a subsequent reaction to fragments with a quinonemethide and a phenolic structure. The results are summarized in table 3 and displayed in figure 3.
Optimized structures can be obtained here:Step 1 (Hydrogen Transfer): I ---> II(TS) ---> III Step 2 (Ether cleavage): III ---> IV(TS) ---> V
Table 1: Calculated state energies for the 1,5-hydrogen
transfer and the subsequent ether bond cleavage. Energies given in kJ/mol.
| Conformer | State | E(State) |
|---|---|---|
| I | S0 | 0.0 |
| T1 | 339.1 | |
| S1 | 424.1 | |
| II (TS) | S0 | 191.2 |
| T1 | 337.0 | |
| S1 | 485.2 | |
| III (Intermediate) | S0 | 134.7 |
| T1 | 165.3 | |
| S1 | 357.4 | |
| IV (TS) | S0 | 188.1 |
| T1 | 321.3 | |
| S1 | 417.3 | |
| V | S0 | 61.6 |
| T1 | 267.2 | |
| S1 | 422.2 |
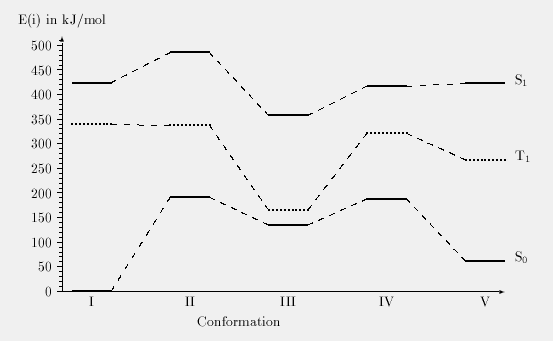 Fig. 3: 1,5-Hydrogen Transfer
Fig. 3: 1,5-Hydrogen Transfer
 Results Overview
Results Overview
 Results ether cleavage
Results ether cleavage