Figure 1: Structures of the studied model compounds DMDPE (left) and DIMEOH (right).

Figure 1: Structures of the studied model compounds DMDPE (left) and DIMEOH
(right).
The obtained absorption spectra for both compounds at two temperatures (293 K and 147 K) are given in figure 2 (load the spectra here for an external viewer). In all cases the photodegradation is clearly reflected in the overall change of the spectra.
At low temperature diffusive processes should become less important due to the increased viscosity of the solvent. But as can be seen, the rate of photodegradation for the compound DMDPE is almost independent of the temperature. Therefore we conclude that the dominant photochemical degradation pathway must be an intramolcular reactions. Intermolecular reactions can be excluded to play a significant role for the compound DMDPE.
In contrast, DIMEOH exhibits a significant temperature dependence. The rate of photodegradation is much larger at room temperature than at lower temperatures. But, one should consider the fact that at 147 K in the spectra two isosbestic points are found. This indicates that only one reaction pathway is possible at this temperature. With increasing temperature the deviation from this simple behaviour gradually becomes larger. This suggests that at higher temperature additional intermolecular pathway become important. We assume that the terminal OH-group is responsible for this different behaviour.
In both compounds a long wavelength absorption between 300 nm and 400 nm appears. This band is responsible for the photoyellowing of the model compounds. We assume that in the polymer similar products are formed. The chinonemethide, one of the products of the intramolecular 1,5-hydrogen transfer, could be responsible for this band.
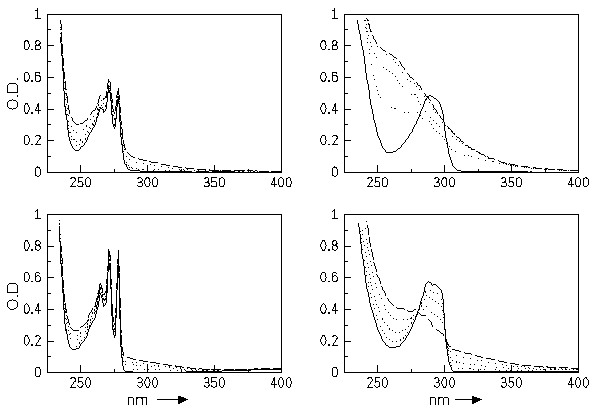
Figure 2: Absorption spectra of DMDPE (left) and DIMEOH (right) in
methylcyclohexane (c=10^-4 mol/l) measured at room temperature, 293 K,
(top) and at 147 K.
Spectra of the samples are given before irradiation (solid lines) and
after irradiating for 5, 10, 20 (dotted lines) and 30 (dashed lines)
minutes.
 Contents
Contents
 Experimental Method -
Conclusions
Experimental Method -
Conclusions
