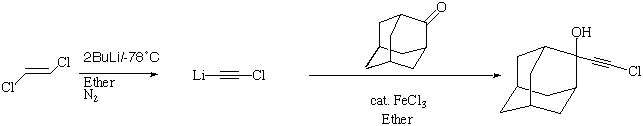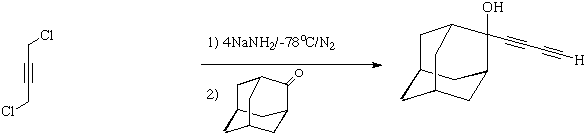Email Discussion:
50 Deposition of x-ray data at Cambridge
Rzepa,Henry
Synthesis and Structural Hydrogen Bonding of 2-ethynyl Adamantane Systems
Michael B. Keller, Henry S. Rzepa, Andrew J. P. White and David J. Williams
Department of Chemistry, Imperial College, London, SW7 2AY.
There is much current interest in probing the diversity and limits of hydrogen bonding interactions
to ¼ systems such as phenyl rings and double and triple bonds. We have previously
identified[1] a small number of molecules for which there is crystallographic evidence for relatively
strong OH...¼ interactions to double or triple bonds. Of these, the most intriguing is the
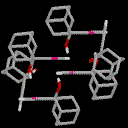
structure of 2-ethynyl adamantan-2-ol 1, which independently
has correctly been identified[1] as
containing both a dimeric intermolecular OH...¼ framework,
with additional C-H...O interactions.
1
As part of our program[2] of
studying ¼-facial interactions, we wished to establish the structural
tolerance of such interactions, and in particular whether simple analogues of
1 might also
demonstrate this effect. We report here the syntheses of five related
ethynyl adamantane derivatives. Although the crystal structure
of 1 has been reported, surprisingly, no details in the literature of a high yield synthesis
are given. In our hands, the simple coupling of ethyne with adamantanone gave
very poor yields, irrespective of the metal counterion used, and we adopted
coupling of the trimethylsilyl alkyne followed by desilylation as the
preferred route. Click on any of the reaction schemes shown below for
active "TGF" files showing the reaction sequences for all the compounds reported here
Full experimental details are also available via hyperlinks to each scheme.
Scheme 1
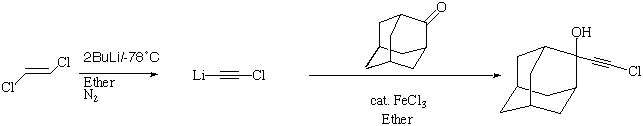
Scheme 2
Scheme 3
Scheme 4
Scheme 5
Results and Discussion.
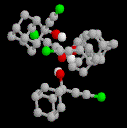
2-chloroethynyl-Adamantan-2-ol: 2
This compound was prepared to investigate the result of suppressing the terminal C-H...O
interaction found in 1. The pattern of hydrogen bonds in this system
changes completely from that found in 1, with the OH interactions to the triple
bond being replaced by a characteristic cyclic tetrameric structure comprising
an 8-membered ring. Clearly, this type of molecule is balanced on a knife edge
between forming O-H..O and O-H...¼ interactions, and any slight change in
the basicity of the alkyne system may perturb sufficiently to radically alter
the hydrogen bonding pattern.
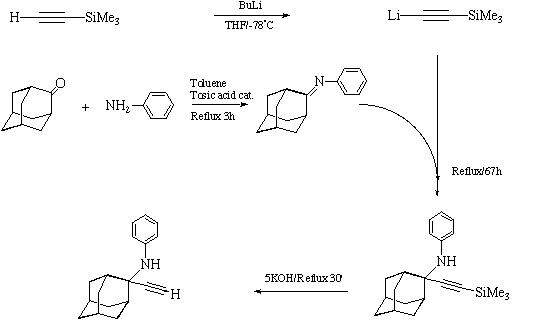
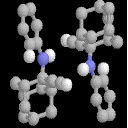
2-ethynyl-2-phenylamino-Adamantane: 3
The phenylamine analogue of 1 exhibits a dimeric NH...¼ interaction
very similar to that found in 1, albeit with significantly longer C...H
distances of around 2.8A rather than 2.2A. There are however two significant
differences in the interactions. A second "ligand" to the triple bond is one of
the ortho hydrogens from the intramolecular phenyl group. Conspicuously, the
terminal C-H hydrogen bond found in 1 appears entirely absent in 3, with
no apparent hydrogen bond forming to the part of the molecule. This might be
a result of greater steric hindrance caused by the phenyl group.
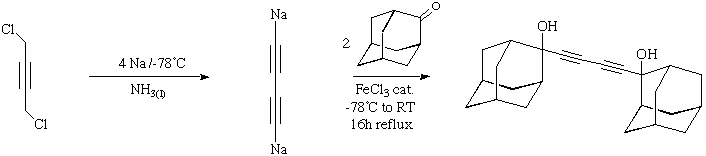
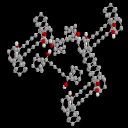
1,4-bis(2-Adamanta-2-ol)-butadiyne: 4
This system represents our first attempt to extend the length of the alkyne
chain to four atoms. In theory, the electron density on such a C4 unit
should promote the basicity of the carbon chain. In practice, the X-ray structure
reverts to the cyclic tetrameric arrangement revealed in compound 2,
with no apparent coordination to the linear C4 fragment. This may in fact
be a consequence of the large bulk of the two "capping" units.
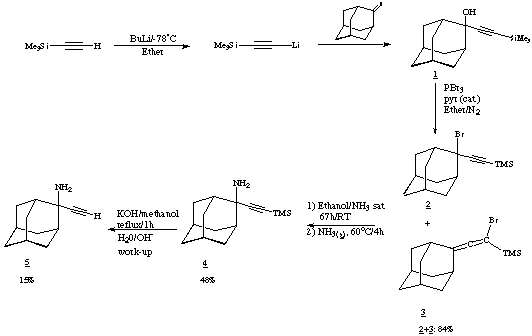
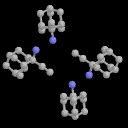
2-ethynyl-2-amino-Adamantane: 5
The amino analogue of 1 exhibits only a terminal C-H...N
hydrogen bonding interaction, and surprisingly no ¼- H-N
interactions. In this it shows a radical departure from the OH system.
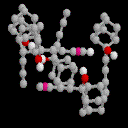
2-butadiynyl-2-Adamantanol: 6
This system shows the closest analogy to 1, with a strong
¼- H-O interaction to the terminal alkyne group rather than the
central one. This interaction is also fairly symmetrical.
Conclusions.
A general theory of the circumstances of how and why ¼- hydrogen bonds
form will require a larger selection of systems than we have yet prepared.
Nevertheless some themes are already emerging. For example, both NH and OH
hydrogen bonds can form. Capping the terminal C-H with a variety of functional
groups such as Cl, SiMe3 (not reported here), phenyl, and adamantanol itself,
appear to suppress ¼ interactions, and initial indications are that extending
the alkyne system to four carbons does not promote the ¼ interactions. It also appears
that a hard/soft acid-base concept might apply in such systems. Thus ¼...H-C
interactions are relatively easy to promote, whereas OH systems revert very
easily to O-H...O interactions. Further structural studies of this system
will be reported in due course.
References
[1] E.Steinwender, E.T.G.Lutz, J.H. van der Maas and J.A. Kanters, Vibrational
Spectroscopy ,
1993, 4, 217.
[2] H. S. Rzepa, M. Smith, and M. L. Webb, J. Chem. Soc., Perkin Trans 2, 1994, 703.
See also
the URL http://www.ch.ic.ac.uk/rzepa/RSC/P2/3_05613l.html