HETEROLYTIC REACTIVITY:
FORMATION OF A QUINONE-IMINE-METHIDE INTERMEDIATE
Hydromethyl substituents have been implicated in the biological activity of various
heterocyclic antitumor drugs such as mitomycin or more recently ellipticine. Their mode
of action can be related to the formation of quinone-methide or quinone-imine-methide
intermediates and the biological importance of such highly reactive species has been
recognized. In most cases, formation of quinone-methide intermediates has only been
postulated for these complex molecules.
Quinone-methides have been extensively studied but there are only few references on
quinone-imine-methides.
We have observed the following reactivity for compound 1:
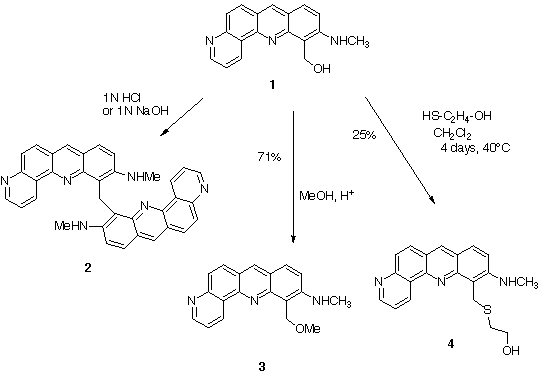 The observed reactivity (dimerisation and addition of various nucleophiles) is in
agreement with a quinone-imine-methide intermediate. We succeeded in trapping this
intermediate (in low yield, 10%) by [4+2] cycloaddition with styrene.
The observed reactivity (dimerisation and addition of various nucleophiles) is in
agreement with a quinone-imine-methide intermediate. We succeeded in trapping this
intermediate (in low yield, 10%) by [4+2] cycloaddition with styrene.



 The observed reactivity (dimerisation and addition of various nucleophiles) is in
agreement with a quinone-imine-methide intermediate. We succeeded in trapping this
intermediate (in low yield, 10%) by [4+2] cycloaddition with styrene.
The observed reactivity (dimerisation and addition of various nucleophiles) is in
agreement with a quinone-imine-methide intermediate. We succeeded in trapping this
intermediate (in low yield, 10%) by [4+2] cycloaddition with styrene.
