HOMOLYTIC REACTIONS:
PHOTOCHEMICAL DEGRADATION
1. REACTION PRODUCT ANALYSIS:
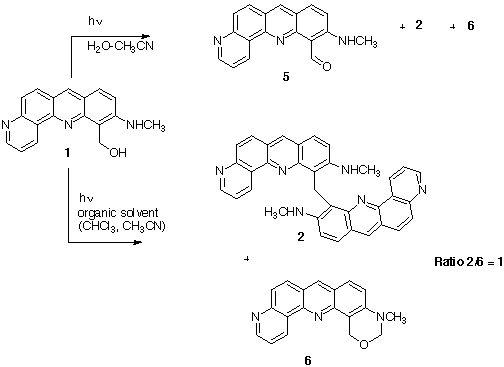
We observed different reaction products when compound 1 was irradiated in the presence
or the absence of water.
In organic solvents (chloroform, methylene chloride, acetonitrile...) two compounds
were isolated and identified: the methylene bis-benzophenanthroline 2 and the N-methyl
dihydro-oxazine 6. These two products were obtained with a 1/1 ratio.
In the presence of water (acetonitrile-water mixtures), the main decomposition product
was identified as the corresponding aldehyde 5.
 2. EPR STUDY:
Due to the low solubility of compound 1 in water, even with a co-solvent, we started the
EPR study of the mechanism of photodegradation in organic solvents.
1) When a solution of 1 in chloroform was irradiated, an EPR spectrum was observed: 8
lines that were attributed to an electron coupling constant with 2 nitrogens and 3 protons
(aN = aH = 5 gauss, g = 2.004 ) (Spectrum A).
We proposed the following structure to this radical:
2. EPR STUDY:
Due to the low solubility of compound 1 in water, even with a co-solvent, we started the
EPR study of the mechanism of photodegradation in organic solvents.
1) When a solution of 1 in chloroform was irradiated, an EPR spectrum was observed: 8
lines that were attributed to an electron coupling constant with 2 nitrogens and 3 protons
(aN = aH = 5 gauss, g = 2.004 ) (Spectrum A).
We proposed the following structure to this radical:
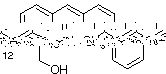 The hyperfine coupling constant will be for the nitrogens in C10 and C12 positions and
the methyl group.
To prove the radical structure, we have prepared compound 1-D in which one hydrogen
of the methyl group has been replaced by a deuterium.
The hyperfine coupling constant will be for the nitrogens in C10 and C12 positions and
the methyl group.
To prove the radical structure, we have prepared compound 1-D in which one hydrogen
of the methyl group has been replaced by a deuterium.
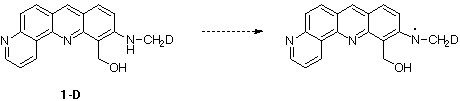 Irradiation of this deuterated analog 1-D in the same conditions gave a different EPR
spectrum with 7 lines attributed to an electron coupling constant with 2 nitrogens and 2
protons, aN = aH = 5 gauss (Spectrum B).
This result is in agreement with the structure that we propose for the observed
radical.
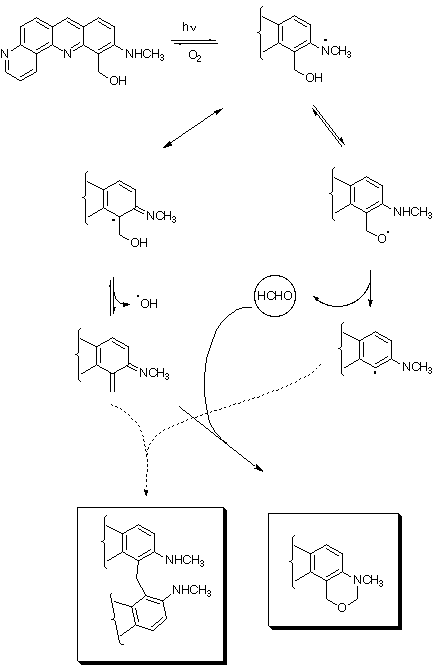
2) Generation of singlet oxygen by compound 1 was shown by a spin trapping
experiment with 2,2,6,6-tetramethyl-4-piperidine.We also demonstrated through spin
trapping measurements with DMPO (5,5-dimethyl-1-pyrrolin-1-oxide) that an superoxide
anion radical adduct was generated (Spectrum C). The addition of a singlet oxygen
scavenger did not decrease the EPR intensity of the adduct. This indicated that singlet
oxygen was not involved in the formation of superoxide anion radical during irradiation
of 1. Generation of superoxide anion radical is due to a particular reactivity of compound
1.
We propose the following working hypothesis, in agreement with the EPR results and
with the 1/1 ratio observed for the formation of the two reaction products 2 and 6:
Irradiation of this deuterated analog 1-D in the same conditions gave a different EPR
spectrum with 7 lines attributed to an electron coupling constant with 2 nitrogens and 2
protons, aN = aH = 5 gauss (Spectrum B).
This result is in agreement with the structure that we propose for the observed
radical.
2) Generation of singlet oxygen by compound 1 was shown by a spin trapping
experiment with 2,2,6,6-tetramethyl-4-piperidine.We also demonstrated through spin
trapping measurements with DMPO (5,5-dimethyl-1-pyrrolin-1-oxide) that an superoxide
anion radical adduct was generated (Spectrum C). The addition of a singlet oxygen
scavenger did not decrease the EPR intensity of the adduct. This indicated that singlet
oxygen was not involved in the formation of superoxide anion radical during irradiation
of 1. Generation of superoxide anion radical is due to a particular reactivity of compound
1.
We propose the following working hypothesis, in agreement with the EPR results and
with the 1/1 ratio observed for the formation of the two reaction products 2 and 6:


 2. EPR STUDY:
Due to the low solubility of compound 1 in water, even with a co-solvent, we started the
EPR study of the mechanism of photodegradation in organic solvents.
1) When a solution of 1 in chloroform was irradiated, an EPR spectrum was observed: 8
lines that were attributed to an electron coupling constant with 2 nitrogens and 3 protons
(aN = aH = 5 gauss, g = 2.004 ) (Spectrum A).
We proposed the following structure to this radical:
2. EPR STUDY:
Due to the low solubility of compound 1 in water, even with a co-solvent, we started the
EPR study of the mechanism of photodegradation in organic solvents.
1) When a solution of 1 in chloroform was irradiated, an EPR spectrum was observed: 8
lines that were attributed to an electron coupling constant with 2 nitrogens and 3 protons
(aN = aH = 5 gauss, g = 2.004 ) (Spectrum A).
We proposed the following structure to this radical:
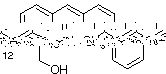 The hyperfine coupling constant will be for the nitrogens in C10 and C12 positions and
the methyl group.
To prove the radical structure, we have prepared compound 1-D in which one hydrogen
of the methyl group has been replaced by a deuterium.
The hyperfine coupling constant will be for the nitrogens in C10 and C12 positions and
the methyl group.
To prove the radical structure, we have prepared compound 1-D in which one hydrogen
of the methyl group has been replaced by a deuterium.
 Irradiation of this deuterated analog 1-D in the same conditions gave a different EPR
spectrum with 7 lines attributed to an electron coupling constant with 2 nitrogens and 2
protons, aN = aH = 5 gauss (Spectrum B).
This result is in agreement with the structure that we propose for the observed
radical.
2) Generation of singlet oxygen by compound 1 was shown by a spin trapping
experiment with 2,2,6,6-tetramethyl-4-piperidine.We also demonstrated through spin
trapping measurements with DMPO (5,5-dimethyl-1-pyrrolin-1-oxide) that an superoxide
anion radical adduct was generated (Spectrum C). The addition of a singlet oxygen
scavenger did not decrease the EPR intensity of the adduct. This indicated that singlet
oxygen was not involved in the formation of superoxide anion radical during irradiation
of 1. Generation of superoxide anion radical is due to a particular reactivity of compound
1.
We propose the following working hypothesis, in agreement with the EPR results and
with the 1/1 ratio observed for the formation of the two reaction products 2 and 6:
Irradiation of this deuterated analog 1-D in the same conditions gave a different EPR
spectrum with 7 lines attributed to an electron coupling constant with 2 nitrogens and 2
protons, aN = aH = 5 gauss (Spectrum B).
This result is in agreement with the structure that we propose for the observed
radical.
2) Generation of singlet oxygen by compound 1 was shown by a spin trapping
experiment with 2,2,6,6-tetramethyl-4-piperidine.We also demonstrated through spin
trapping measurements with DMPO (5,5-dimethyl-1-pyrrolin-1-oxide) that an superoxide
anion radical adduct was generated (Spectrum C). The addition of a singlet oxygen
scavenger did not decrease the EPR intensity of the adduct. This indicated that singlet
oxygen was not involved in the formation of superoxide anion radical during irradiation
of 1. Generation of superoxide anion radical is due to a particular reactivity of compound
1.
We propose the following working hypothesis, in agreement with the EPR results and
with the 1/1 ratio observed for the formation of the two reaction products 2 and 6:
