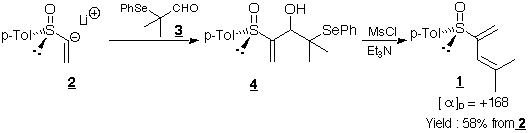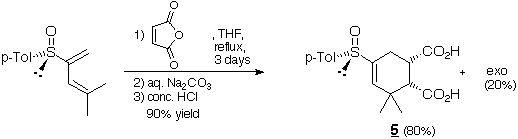Email Discussion:
http://www.ch.ic.ac.uk/ectoc/papers/gosselin/
d.craig@ic.ac.uk
Email Discussion:
Paper 31 (forwarded for Dr. Yannick LANDAIS)
Dr J M Goodman
Email Discussion:
Re: Paper 31 (forwarded for Dr. Yannick LANDAIS)
Pascal GOSSELIN


Diastereoselectivesynthesis Of (-)-Karahana Ether By Diels-Alder Reaction Of
Enantiopure(+)-(R)-4-Methyl-2-P-Tolylsulfinyl-1,3-Pentadiene.
C. Maignan, E. Bonfand and P. Gosselin*
Laboratoire de Synthèse Organique, URA CNRS 482,Faculté des
Sciences, Université
du Maine, 72017 Le Mans CEDEX,France.
E mail: gosselin@univ-lemans.fr
Diene 1 was prepared bydirect condensation of lithium
(+)-(R)-p-tolylvinylsulfoxide 2 on
α-phenylselanylisobutyraldehyde3 and subsequent Krief-Reich
anti-elimination on the resultingsulfinyl-β-hydroxyselenide
4.
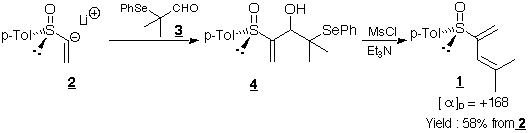
Diene 1 is apotentially useful precursor of
natural terpenoids. As anillustration, the total stereoselective synthesis
of (-)-(1S,5R)-Karahanaether 10 was realized by mean of an
asymmetric Diels-Alder reaction as thekey-step:
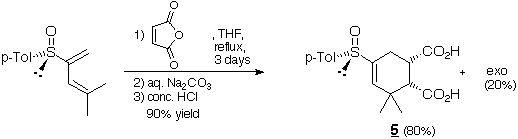
The main hydrolyzed cycloadduct 5 resulted probably from an endo
approach of maleic anhydride bythe less sterically hindered side of diene
1 (opposite to the p-tolyl group) which is also the most
nucleophilic one.This indicates a s-trans conformation of the S=O and
C=Cbonds in compound 1, at least in the transition state (as
previously reported in the case ofanother 2-sulfinyldiene :Tetrahedron :
Asymmetry 5 (5) 781-4 1994 ).
Enantiopure diol 7 wasobtained via a two steps reduction.
This process allowedthe complete separation of intermediate lactones
6a +6b from their exo-diastereomers.
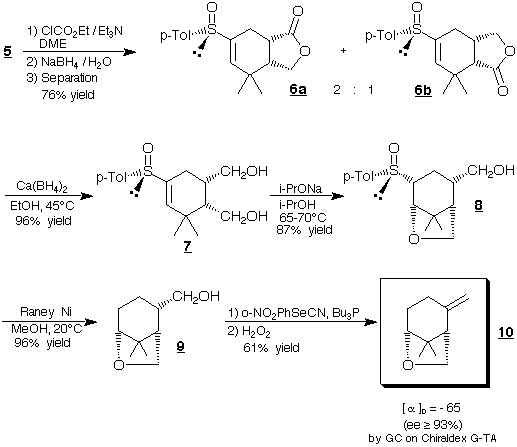
Heterocyclization to ether 8 was realized by an intramolecular
conjugate addition of the solepseudo-axial heteroanion. Further hydrogenolysis
and deshydratation of8 gave natural (-)-(1S,5R)-Karahana ether
10 (33% yieldfrom 1).
This synthesis utilizes both main properties of the sulfinylgroup :
* good chiral induction agent in cycloaddition
* efficient activating group in Michaël addition.