The use of microorganisms, such as bakers' yeast, for bio-reduction processes will also become more widespread. The rate of expansion of this area of biotransformations will be slower due to the inevitable problems associated with the use of whole-cell systems ( Roberts, et al., 1995). The employment of dehydrogenase enzymes for the reduction or oxidation of organic substrates will also become more attractive to the synthetic organic chemist in the wake of further refinement of cofactor recycling systems.
More research work is needed on the use of enzymes for other oxidation reactions (Baeyer-Villiger type reactions, controlled hydroxylation etc.) before more extensive use is made of these catalysts. In five years time the situation should be different.
Similarly the use of enzymes for the stereocontrolled formation of carbon-carbon bonds will need more attention in research laboratories before the full potential of these catalysts is realized. The exceptions are the enzymes that catalyse the stereoselective formation of cyanohydrins which are now available for more routine use.
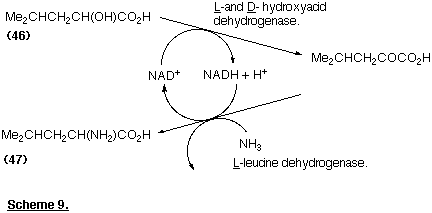
The possibility of setting up a cascade of enzyme-catalysed reactions in the same "pot" is very attractive. The conversion of the racemic hydroxy acid (46) into the amino-acid (47) illustrates the sort of coupled process that is feasible (Scheme 9). (Schmidt-Kastner and Egerer, 1984): note that co-factor recycling is accommodated within this sequence.

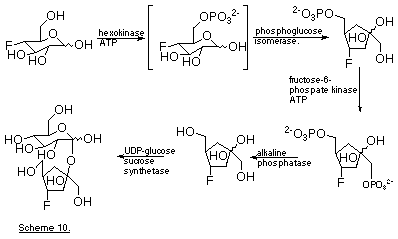
The preparation of unusual sugars, and sugar analogues, using enzyme-catalysed processes will gain prominence (Toone et al., 1989). The work of Card et al. (1986) (Scheme 10) illustrates the elegant way in which fairly complex end-products can be built up without the need for extensive protection/deprotection sequences.
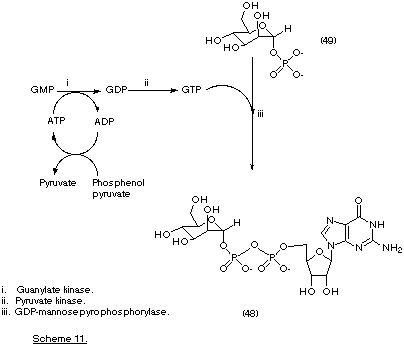
An analogous multi-enzyme approach has been applied to the synthesis of the nucleotide coenzyme GDP-mannose (48) (Scheme 11). In this reaction the GTP is initially prepared from GMP using cofactor recycling. The GTP thus formed can then be combined with mannose-1-phosphate (49) in the presence of GDP-mannose pyrophosphorylase to yield the product ( Pallanca and Turner, 1993).
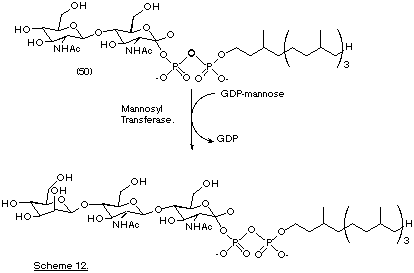
In an extension of this work, those enzymes that utilise GDP-mannose as a source of mannose are also being studied ( Pallanca and Turner, 1993). These mannosyl transferases catalyse the transfer of mannose to lipid linked substrates thus generating complex mannosyl containing oligosaccharides that are important constituents of natural N-linked glycoproteins. It has been shown that the novel lipid-linked substrate (50) can act as an efficient acceptor for mannose transfer thereby opening the way for the use of this approach to synthesise oligosaccharides on a preparative scale (Scheme 12) (Flitsch et al., 1991).
Progress in the field of biotransformations will be matched by equally exciting advances in the area of synthetic organic chemistry. The advances will often be complementary and the use of enzymes for the preparation of materials which are difficult to make by purely chemical methods will remain in vogue for the foreseeable future. Certainly the present problems with the disposal of waste organic solvents, with the consequent focus on reactions that can be conducted in an aqueous environment, concentrate attention on Nature's catalysts which are designed to operate in aqueous solution. Finally it is conceivable that "man-made" enzymes (i.e. catalytic antibodies) will also make a significant impact on research in this area and add to the range of organic reactions that can be catalysed by protein ( Kang et al., 1990).
In conclusion the next ten years should see a lot of exciting developments in the area of biotransformations. Many of the forthcoming investigations will be aimed at producing bulk quantities of optically active, relatively simple molecules, while an equal volume of work will concentrate on the preparation of more complex, higher value materials in smaller quantities. It promises to be a fascinating decade for those scientists involved in the research and development of processes that have enzyme catalysed reactions as the key step(s).