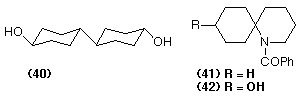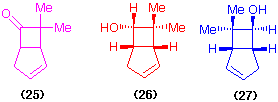
Carbonyl Reductions
Partially purified dehydrogenase enzymes have also been utilised for the production of optically active secondary alcohols. During the purification of the protein the enzyme cofactor (nicotinamide adenine dinucleotide (phosphate) [NADPH]) is lost and so before the biotransformation is possible this cofactor must be re-coupled to the enzyme. The cofactors NADH and NADPH are expensive and cofactor recycling must be set up so that a less-than-stoichiometric amount of the dinucleotide can be employed. Cofactor recycling systems are now well defined thanks to the work of J. Bryan Jones (1986) and others, and are ready for use in non-specialist laboratories.
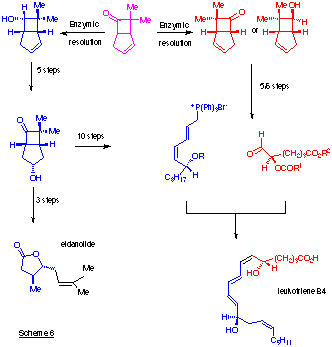
The ketone (25) has been reduced using Mortierella spp. and by 3ã,20ß-hydroxysteroid dehydrogenase/NADH. Thus the whole cell reduction gave an equimolar mixture of the 6endo-alcohol (26) and the 6exo-alcohol (27) (60% yield of diastereoisomeric alcohols of high optical purity (Cotterill et al., 1991) while the dehydrogenase catalysed an enantioselective reduction of the ketone (25) by NADH, forming the alcohol (26) in an optically pure state and leaving optically active ketone. The alcohol (26) has been converted into eldanolide, the pheromone of an agricultural pest, the sugar cane borer (Scheme 6). The availability of the two enantiomers of the 7,7-dimethylbicyclo[3.2.0]heptane ring system allows the synthesis of biologically interesting leukotriene-B4 (Cotterill et al., 1991).
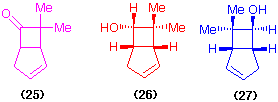
Note that one enantiomer of the ketone provides the C1-C6 section of the natural product (containing one chiral centre while the other enantiomer can provide the C7-C20 portion (containing the second chiral centre) (Scheme 6) (Cotterill et al., 1990; Dormán et al., 1989).
Reduction of Aromatic Nitro Groups

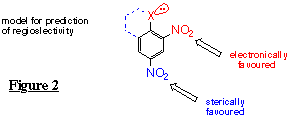
Oxidation of aromatic and alicyclic molecules using monooxygenase and dioxygenase enzymes has attracted a considerable amount of attention.
Dioxygenases
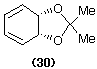
For example various cycloaddition reactions can be carried out on the acetonide (30), including Diels-Alder [4+2] reactions [to give, for example, the tricyclic compound (31)], [2+2] cycloaddition reactions [to give cyclobutanone derivatives such as (32)] (Pittol et al., 1994) and the more unusual [6+4] cycloaddition reactions (Cotterill et al., 1988b; Mahon et al., 1991).
Not only is benzene biotransformed by Pseudomonads but so are many of its derivatives. Often the latter compounds are obtained in an optically pure form and it is established (at least for some of the compounds) that these diols have the 1(S),2(R)-configuration.
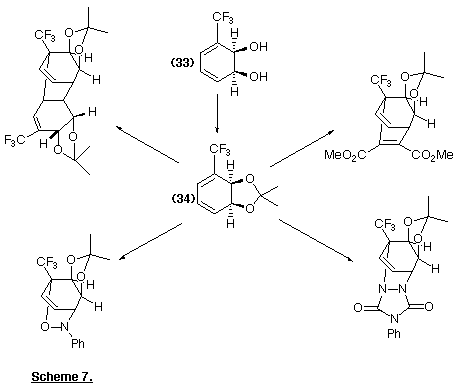
Trifluoromethylbenzene is biotransformed into the diol (33); the corresponding acetonide (34) can be converted into a wide range of derivatives (Scheme 7) through different chemical reactions (Pittol et al., 1989; Downing et al., 1990).
Interesting conversions involving 3-chlorocyclohexa-3,5-diene-1,2-diol and 3-methylcyclohexa-3,5-diene-1,2-diol have also been described (Hudlicky et al., 1989; Carless 1992).
Monooxygenases
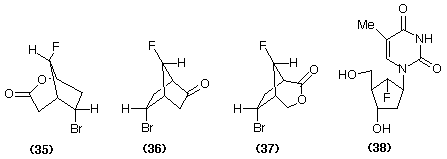
The monooxygenase from the Acinetobacter can be purified and Baeyer-Villiger oxidations can be performed using the enzyme in association with its cofactor NADPH. We have found that it is possible to convert a secondary alcohol into the corresponding lactone via the corresponding ketone by using a coupled enzyme system, that is Thermoanaerobium brockii dehydrogenase and A. calcoaceticus monooxygenase with in situ recycling of the cofactor (Scheme 8) (Willetts et al., 1991). More recently a monooxygenase enzyme capable of using NADH as cofactor has been found, enabling cofactor recycling to be accomplished using formate dehydrogenase (Grogan et al., 1993, Gagnon et al., 1994).
As adumbrated previously, immobilised soya bean lipoxygenase-1 efficiently converts linoleic acid into 13(S)-HODE (3) in 78% yield in a few hours. The immobilised enzyme can be recovered and reused. The chemistry of 13-HODE has been investigated: for example the bicyclic compound (39) has been obtained by macro-lactonization and a Diels-Alder reaction on the biotransformation product (Maguire et al., 1994).
The ability to derivatize an organic molecule at a position distant from pre-existing functionality is an attractive goal for both organic chemists and microbiologists alike. Some progress has been made towards providing chemical methods for remote hydroxylation (Sarneski et al., 1991) but, for the foreseeable future, microbiological methods seem to offer the most likely chances for success. The regioselective hydroxylation of steroids has been investigated in some depth, the formation of 11-hydroxyprogesterone from progesterone being one of the most noteworthy successes. The regio-selective (but not regiospecific) oxidation of cyclohexylcyclohexane to give the diol (40) using Cunninghamella blakesleeana shows that even the most hydrophobic materials can be biotransformed (Davies et al., 1986). Spiro-heterocyclic compounds can be derivatized, often in very high yield, to produce single compounds using Beauveria sulfurescens: for example the piperidine derivative (41) furnishes the alcohol (42) in 70% isolated yield (Floyd et al., 1993).