The Results
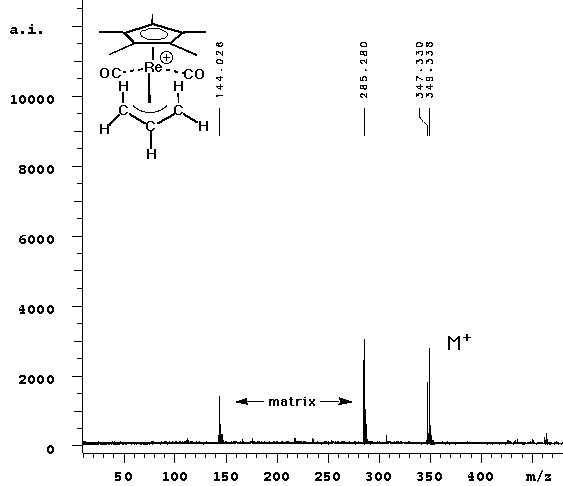
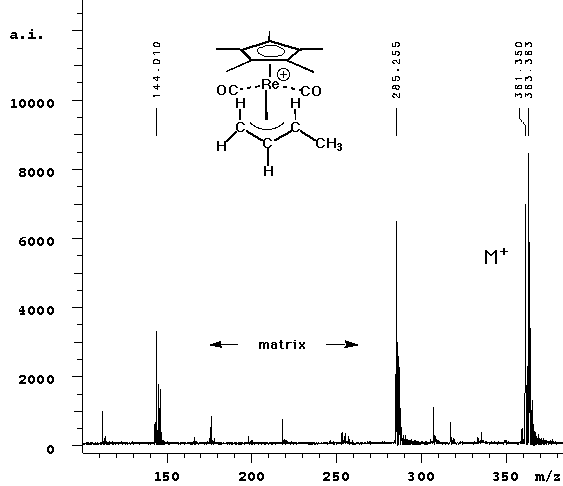
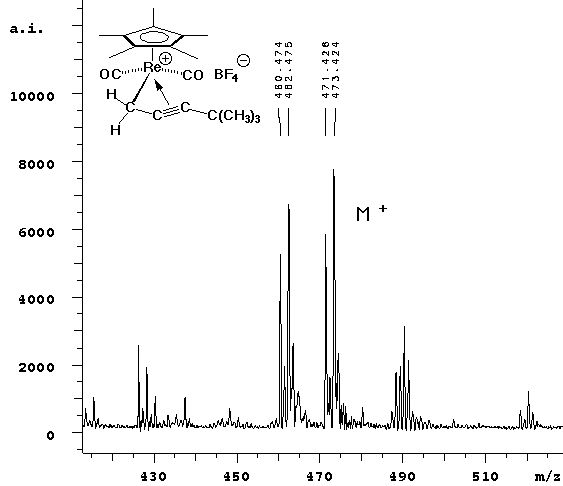
| In this experiment, we observed the parent peak (calcd 347.02, obsd 347.33) in cation detection mode. No fragmentation was observed -- the two lower mass peaks are from the ATT matrix and its dimer. |
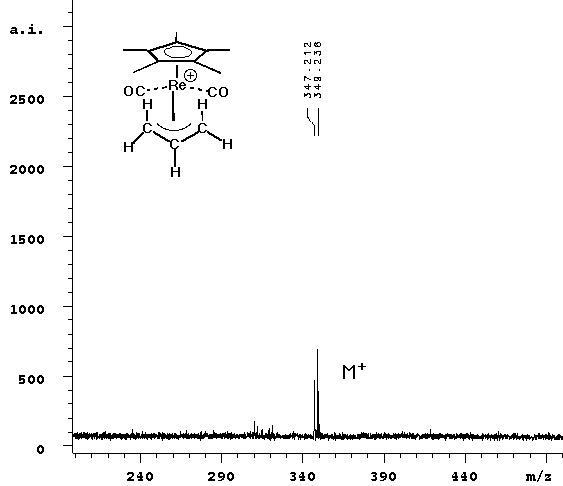
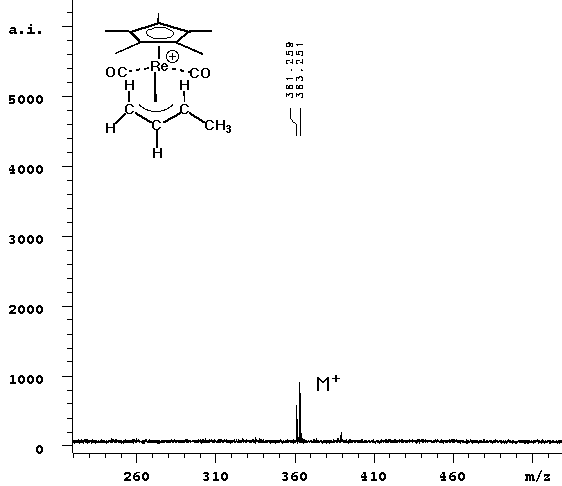
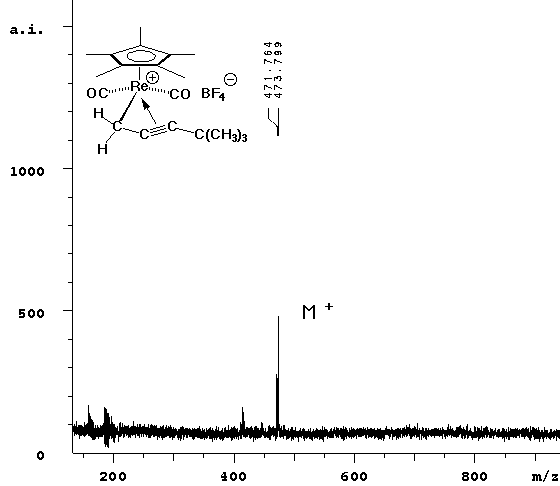
| The experiment was repeated without any matrix. Since cyclopentadienyl rhenium complexes absorb light over a wide range of energy, it seemed reasonable that they could be ejected from the surface in the same way that standard matrices are. The laser power required to see signal was slightly higher without the ATT matrix, but the result (right spectrum above) cleanly showed the parent ion only. |