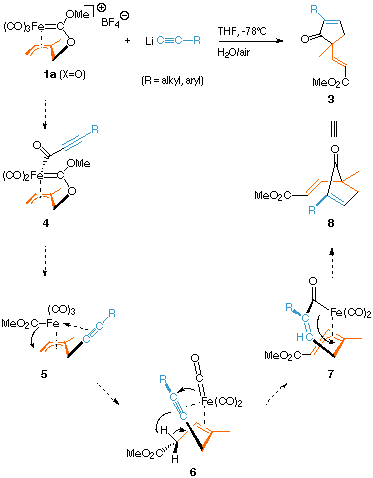

Whereas "soft" nucleophiles like enolates, enoxy borates and cuprates attack the allyl–ironcarbene complexes 1 and 2 preferentially at the terminal carbon atom C-6 (see path B for example), lithium acetylides react differently and form functionalized cyclopentenones[5]. The mechanistic details of this unprecedented reaction are not entirely clear yet. The first step appears to be the formation of acylcarbene intermediate 4 by attack of the acetylide on an ancillary carbonyl ligand. Complexes 4 can be isolated in good yields as yellow crystalline compounds by chromatography of the unhydrolyzed reaction mixtures at -40 °C (silica gel; ether). They are stable under inert atmosphere at this temperature and can be characterized by NMR-spectroscopy but quickly and irreversibly decompose upon warming up to 0 °C. None of the other follow-up intermediates of the speculative proposal depicted in the scheme above could be trapped or characterized so far, but at least semiempirical calculation methods (AMPAC5.0/SAM1, SINDO1/ROHF and ZINDO/UHF) support them as reasonably low in energy. To account for the connectivity of carbon atoms in 3 we assume a shift of the acetylide moiety from the CO ligand to the homoallylic position C-3 with cleavage of the C-3–O-2 bond to give the intermediate neutral (methoxycarbonyl)–allyliron complex 5. This species could immediately rearrange by transfer of the ester group to the allyl terminus C-6 with formation of complexes like 6; this process is facilitated by coordination of the alkyne unit to the iron atom. Calculations show a low-energy trajectory leading from 6 to the metallacyclic acylallyliron complex 7 through simultaneous CO insertion and proton transfer from the former C-6 to the acetylene ligand, which restores the eta3-allyl system. Oxidation (by air) or hydrolysis of 7 would then yield product 3 by demetalation/cyclization.
This cascade process furnishes four new C–C bonds with concomitant insertion of one CO unit and a formal transfer of the carbene function as a methoxycarbonyl group to the former allyl terminus C-6. 3 is predominantly (GC > 95%) formed as the isomer with E configuration of the exocyclic double bond and with the alkyl residue of the starting acetylene next to the carbonyl group of the cyclopentenone ring. These reactions still require optimization and improvement in the yields but appear quite promising for application to natural cyclopentenone synthesis due to their mild reaction conditions, selectivity, and "one-pot"-, multi–step character.
Experimental data
Synthesis of 3 (general procedure):
A solution of the 1-alkyne ( 1.20 mmol) in 2 ml of THF was cooled to -78°C and then treated with nBuLi (0.48 ml of a 2.5M solution in hexane; 1.20 mmol). The resulting mixture was stirred at this temperature for 15 min and finally at room temp. for a further 60 min. After recooling to -78 °C it was slowly transferred with a cannula to a slurry of 1a (350 mg; 1.00 mmol) in 5ml of THF at -78 °C. The reaction was stopped by the addition of one equivalent of a saturated aqueous solution of NH4Cl after another hour at -78 °C. The mixture was then repeatedly extracted with ether and the combined organic extracts were dried. The solvent was evaporated and the residue purified by column chromatography (petroleum ether/ether, 5:1).
(±)-[5-((E)-2-methoxycarbonyl-ethen-1-yl)-5-methyl-2-pentyl-cyclopent-2-en-1-one] (3a):
IR (neat): 2940, 2910, 2840, 1710, 1640, 1620, 1430, 1310, 1270, 1190 cm-1.-1H NMR (CDCl3, 400 MHz): d = 0.85 [t, 3J (15-H/14-H) = 6.90 Hz, 3H, 15-H], 1.27 - 1.34 [m, 7H, 10-H, 13-H, 14-H], 1.43 - 1.52 [m, 2H, 12-H], 2.17 [dt, 3J (11-H/12-H) = 7.7 Hz, 4J (11-H/3-H) = 1.5 Hz, 2H, 11-H], 2.52 [dq, 4J (4-Hup/10-CH3) = 2.3 Hz, 2J(4-Hup/4-Hdown) = 18.8 Hz, 1H, 4-Hup], 2.77 [dq, 4J (4-Hdown/10-CH3 ) = 2.3 Hz, 2J (4-Hup/4-Hdown) = 18.8 Hz, 1H,
4-Hdown], 3.72 [s, 3H, 9-H], 5.89 [d, 3J (6-H/7-H) = 15.9 Hz, 1H, 7-H], 6.87 [d, 3J (6-H/7-H) = 15.9 Hz,1H, 6-H], 7.27 [m,1H, 3-H]. - 13C NMR (CDCl3,100.5 MHz): d = 14.05 (C-15), 22.38 (C-14), 22.87 (C-10), 25.07 (C-11), 27.28 (C-12), 31.53 (C-13), 41.66 (C-4), 49.71 (C-5), 51.59 (C-9), 120.16 (C-7), 144.37 (C-2), 150.33 (C-6), 154.23 (C-3), 166.86 (C-8), 208.64 (C-1). - MS (70 eV); m/z (%): 250 (50) [M+], 235 (10) [M+ - CH3], 219 (18) [M+- OCH3], 191 (100) [M+- COOCH3], 175 (8) [{191} - CH4], 161 (38), 133 (22), 112 (18), 105 (21), 80 (38). - C15H22O3 (250.3): calcd. C 71.97, H 8.86; found C 71.90, H 8.82.