Probable direction of electrophilic attack
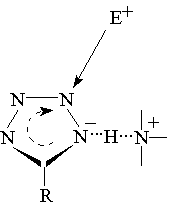 |
Two examples of reactions of triethylamine tetrazolate with electrophilic reagens
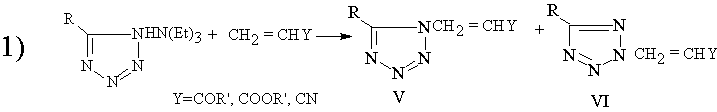 |
In the case that R=Ar or another bulky groups , the N2-isomer (YI) is obtained primarely [1,2].
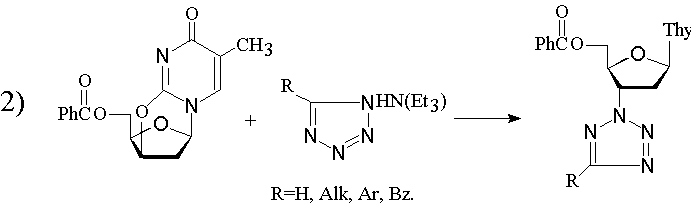 |
In this untypical case only N2-isomers for all R have been obtained [3].
We are studying now a the mechanism of this process by kinetic methods.