 |
Akylation of Tetrazoles and Tetrazolium Cations by Carbonium Ions Generated from Alcoholes
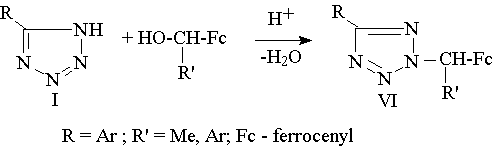
Regioselective alkylation of 5-aryltetrazoles with a -methyl- and a -arylferrocenyl alcoholes in acetic acid medium [1]
 |
Regioselective alkylation of 5-substituted tetrazoles with isopropyl alcohole in sulfuric acid medium [2,3]
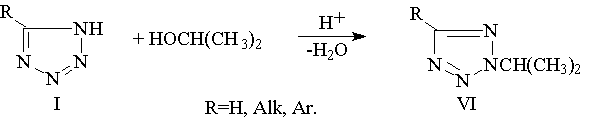 |
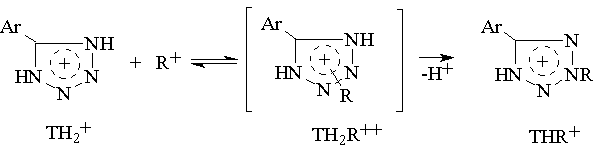
An analysis of kinetic data shows that a transition state of this reaction is protonated tetrazole.
 |
Values of the true rate constants k were calculated by the equation:
k = kobs ((1 + 1/It) (1 + aw KR+ (1 + 1/Ia)) ,
where It, Ia are ionization ratios of the corresponding 5-aryltetrazole and isopropyl alcohol, respectively; KR+ = -5.30 is the equilibrium constant of isopropyl cation formation from protonated isopropyl alcohol; and aw is water activity.