 |
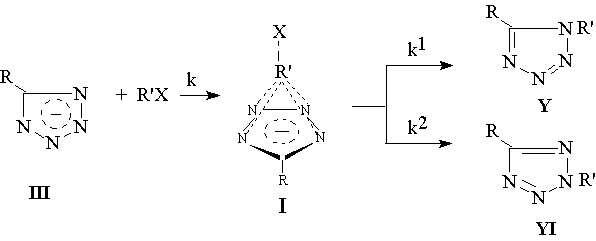
The Mechanism of Tetrazolate Alkylation
The reaction mechanism of tetrazolate (III) alkylation is proved to be two-stage one. The reaction fast and rate limiting stage is an interaction between the anion and alkylation agent. The result of this interaction is formation of active intermediate (I). And on the second stage the rapidly reogranization of intermediate (I) lead to isomeric N1- and N2-tetrazoles (Y,YI) formation [1-7].
 |
lgF = (E1-E2) / 2.3RT + lgA2/A1; F= [YI]/[Y]= k2/k1;
M.L.Shpak,V.A.Ostrovskii, I.Yu.Shchirobokov,G.I.Koldobskii, B.V.Gidaspov, Zh.Org.Khim. 14, ? 11, 2444-2446 (1978).
I.Yu.Shchirobokov, V.A.Ostrovskii, G.I.Koldobskii, Zh.Org.Khim. 15, ? 4, 839-844 (1979).
I.Yu.Shchirobokov, V.A.Ostrovskii, G.I.Koldobskii, Zh.Org.Khim. 16, ? 4, 788-792 (1980).
I.Yu.Shchirobokov, V.A.Ostrovskii, G.I.Koldobskii, Zh.Org.Khim. 16, ? 10, 2145-2149 (1980).
V.A.Ostrovskii, I.Yu.Shchirobokov,G.I.Koldobskii, Zh.Org.Khim. 17, ? 1, 146-151 (1981).
L.N.Agarkova, V.A.Ostrovskii, G.I.Koldobskii, G.B.Erusalimskii Zh.Org.Khim. 18, ? 5, 1043-1047 (1982).
V.S.Poplavskii, I.E.Titova, V.A.Ostrovskii, G.I.Koldobskii, Zh.Org.Khim. 18, ? 9, 1081-1085 (1982).