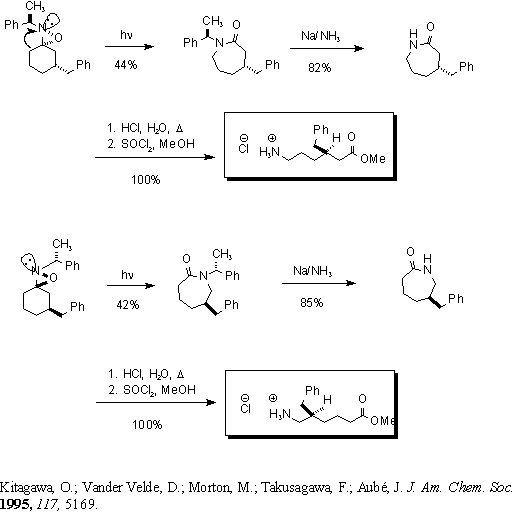
Upon photolysis,
the mixture of oxaziridines (shown separately here) undergoes a stereoselective
ring expansion to yield a diastereomeric mixture of lactams, which are
separated by column chromatography. It is hypothesized that the bond
antiperiplanar to the lone pair of electrons on the nitrogen migrates during
the rearrangement. A Birch reaction is performed on each lactam to
remove the phenylethyl moieties to afford enantiomeric lactams. The
3- and 5-benzyl-Aca linkers are obtained after hydrolysis of each lactam
and formation of the corresponding methyl ester.
 Back to Index
Synthesis of the 4-Benzyl-Aca Linker
Stereochemistry of Lactam Formation
Back to Index
Synthesis of the 4-Benzyl-Aca Linker
Stereochemistry of Lactam Formation

