2.
EXPERIMENTAL PROCEDURES
2.1.
Materials
E. coli (CR 261) was a gift from Dr. Ch.
Roessner of Texas A & M University. Production of E. coli and
purification of PBGS has been described earlier [20]. Compounds
16, 17 ,18 ,19 ,20 ,21 ,23 ,24 and 29 were bought from Fluka and compound
22 from Aldrich. All the other inhibitors rac-25 [47-49], rac-26
[47-49], 27 [50], 28 [51,52], 30 [52,53],
31 [54], 32 [55], 33 [56] and 35 [57] were
synthesized in our laboratories following the corresponding references.
Synthesis of 5-(3-nitropropionylamino)pentanoic
acid (34)
In a two necked round-bottom flask were introduced
under N2, 1.07 g (9 mmol) 3-nitroproionic acid, 40
ml dichloromethane and 2 drops of dimethylformamide. The suspension was
cooled with an ice-bath and 1.26 g (9.9 mmol) oxalyl chloride was added
over 5 min. After 3 h at r.t. the solution was cooled with an ice-bath
and 2.73 g (9 mmol) 4-methoxycarbonylbutylammonium toluene-4-sulfonate
and 911 mg (9 mmol) triethylamine in 25 ml dichloromethane were added dropwise
followed by 911 mg (9 mmol) triethylamine neat over 15 min. After 2 h,
the ice-bath was removed and the reaction was kept at r.t. for 1 h then
quenched with 60 ml 1 M HCl. The aqueous phase was extracted with 60 ml
dichloromethane and the combined organic phases were washed with 60 ml
KHCO3 10%, 60 ml brine, dried over MgSO4
anhydrous, filtered and evaporated. The resulting oil (1.55 g) was separated
by flash column chromatography (dichloromethane with 1 to 4% methanol)
to obtain 1.33 g (64%) white solid methyl 5-(3-nitropropionylamino)pentanoate.
C9H16N2O5
(232.24) C 46.72 (46.55), H 7.18 (6.94), N 12.35 (12.06); Rf
(CH2Cl2-MeOH 9 : 1, KMnO4)
= 0.62; m.p. = 50-51°C; IR (KBr) : 3302m, 3105w, 3031w, 2957w,
2924m, 2854w, 1733s, 1646s, 1552vs, 1446m, 1413m, 1370m, 1226m, 1202m,
1165m; 1H-NMR (400 MHz, CDCl3) :
1.49-1.56 (m, 2H, H2C(4)); 1.60-1.67 (m,
2H, H2C(3)); 2.32 (t, 3J2-3
= 7.2, 2H, H2C(2)); 2.80 (t, 3J7-8
= 6.2, 2H, H2C(7)); 3.04 (td, 3J5-4
~ 6.6, 3J5-HN ~
5.6, 2H, H2C(5)); 4.69 (t, 3J8-7
= 6.2, 2H, H2C(8); 6.22 (s(br), 1H,
HN); 13C-NMR (100 MHz, CDCl3,
HETCOR) : 21.8 C(3); 28.7 C(4); 32.5 C(7); 33.3 C(2); 39.2 C(5); 51.5
C(9); 70.2 C(8); 168.3 C(6); 173.9 C(1); MS (DCI) : 250 (29, [M+18]+),
233 (100, [M+1]+), 201 (8, [M-CH3O]+),
100 (15, [C5H10NO]+).
In a 100 ml Erlenmeyer flask were introduced 1.2
g (5.17 mmol) methyl 5-(3-nitropropionylamino)pentanoate, 50 ml water and
3 mg hog liver esterase in 0.3 ml 3.2 M ammonium sulfate. For 4 h, pH =
7.3 was kept by pH-state controlled adding of 0.5 M NaOH, then the solution
was acidified with conc. HCl, saturated with NaCl and extracted 4 times
with 50 ml ethyl acetate. The combined organic phases were dried over MgSO4,
filtrated and evaporated. The resulting orange solid (1.01 g) was crystallized
from tetrahydrofurane and diethylether to obtain 810 mg (72%) white solid
5-(nitropropionylamino)pentanoic acid.
C8H14N2O5
(218.21) C 44.24 (44.03), H 6.53 (6.47), N 12.67 (12.84); Rf
(ethyl acetate-MeOH 2 : 1, KMnO4) = 0.52; m.p. = 82-83°C;
IR (KBr) : 3298s, 3071m, 2948m, 2878m, 1692s, 1638s, 1551vs, 1477m,
1464m, 1416m, 1377m, 1334m, 1280m, 1218m, 1192m, 924m, 872m, 681m; 1H-NMR
(400 MHz, d8-DMSO) : 1.35-1.42 (m, 2H,
H2C(4)); 1.45-1.52 (m, 2H, H2C(3));
2.20 (t, 3J2-3
= 7.3, 2H, H2C(2)); 2.73 (t, 3J7-8
= 6.0, 2H, H2C(7)); 3.04 (td, 3J5-4
= 7.0, 3J5-HN = 5.3, 2H,
H2C(5)); 4.68 (t, 3J8-7
= 6.0, 2H, H2C(8); 8.04 (t, 3JHN-5
= 5.3, 1H, HN); 12.00 (s, 1H, HO); 13C-NMR
(100 MHz, d8-DMSO, HETCOR) : 21.9 C(3); 28.5
C(4); 31.4 C(7); 33.2 C(2); 38.2 C(5); 70.7 C(8); 168.3 C(6); 174.4 C(1).
2.2.
PBGS Assay and Determination of Kinetic Constants
The PBGS assay is a colorimetric assay based on
the reaction between PBG and 4-dimethylaminobenzaldehyde [3].
The assay for E. coli PBGS contained 4
- 6.4 µg PBGS, the inhibitor in 1.5ml of 0.1M KP, at pH=8.1,
12.3 mM mercaptoethanol, 10 mM MgCl2.6H2O and 10 µM ZnCl2. The preincubation
took place at 37° for 30-45 minutes. The substrate was added in varying
concentrations and the solution was incubated for 14 minutes. After the
14 minutes of incubation the PBGS-catalysed reaction was stopped by 1 ml
of the stop reagent (20% TCA, 10 mM HgCl2) at 0°C. After centrifugation
(4 min, 1900 g) 1 ml of the supernatant was treated with 1 ml of Ehrlich's
reagent (4-dimethylaminobenzaldehyde in perchloric acid 70% / acetic acid
solution). This solution was centrifuged (4 min, 1900 g) for a second time.
The quantity of product formed was determined by measuring the absorbance
at 554 nm (e =
60'000 [mol-1 * cm1]).
The type of inhibition was determined using Eadie-Hofstee,
Lineweaver Burk and Hanes plots. The Ki shown in the table 1 and table
2 are the average of at least three independent assays. Ki-values were
calculated from the Kmapp deduced from the hyberbola plot, typical for
Michaelis-Menten kinetics.
2.3.
Estimation of the specific activity
The specific activity is estimated by the Bio-Rad
Protein Assay using the color change of the Coomassie Brilliant Blue-G-250.
This color change is followed by measuring the absorbance at 596 nm. The
enzyme quantity is deduced by comparison with a standard curve.
2.4.
Dialysis Assay
4 - 6.4 µg PBGS were dissolved in 10 ml
phosphate buffer (0.1M KP, pH=8.0, 12.3 mM mercaptoethanol, 10 mM
MgCl2.6H2O and 10 µM ZnCl2). 4 ml of this solution were placed in
two different 5 ml tubes, one containing 5.8 mg of 4-oxo-sebacic acid (30)
and the second (without inhibitor) was used as a reference. After 24 hours
at room temperature, the specific activities were determined. The solution
containing the inhibitor showed only 22.4% of the specific activity compared
to the blank experiment.
1 ml of each solution is placed in a dialysis
tube and were seperately dialysed against 2.5 l phosphate buffer solution
(0.1M KP, pH=8.0, 12.3 mM mercaptoethanol, 10 mM MgCl2.6H2O
and 10 µM ZnCl2). After 66 hours dialysis at
4°C, the specific activities were again determined (the solution containing
the inhibitor showed 28.5% of the specific activity compared to the blank
experiment).
The same measures were repeated with the compound
(35).
Go to main
menu
3.
RESULTS
3.1.
Inhibition of E. coli PBGS with Diacids
A series of diacids were tested as potential analogues
of the intermediates. We hoped thereby to obtain further hints on the mechanism
of the PBG biosynthesis.
Almost all of the diacids tested showed competitive
behaviour (Table 1). The inhibition constants of the competitive
inhibitors are ranging from values close to 10'000 µM to more then
20'000 mM for the
2-nitro benzoic acid (20) and the compound 32. These competitive
inhibitors all show only a moderate inhibition potency. It is interesting
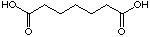
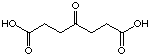
to compare pimelic acid (23) and 4-oxo pimelic acid (24).
The introduction of a keto group in position 4 in pimelic acid is lowering
the Ki-value from 12'400 µM to 8'600 µM.
Much to our surprise there is no significant difference as expressed by
the inhibition constant between maleic acid (18) and fumaric acid
(17). Even the more sterically demanding phthalic acid (19)
is in the same range of inhibition.
The most evident difference is observed between
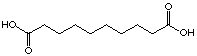
sebacic acid (29) and the 4-oxo sebacic acid (30). The introduction
of the keto function did not only improve the Ki value,
like it was the case for pimelic acid, but a change of the type of inhibition
occured. Sebacic acid (29) showed a moderate competitive inhibition
(Ki=7'975 mM)
whereas the 4-oxo-sebacic acid (30) became an irreversible inhibitor
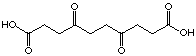
just due to the introduction of the keto function. Others cases of irreversible
inhibition were found for the compounds 35 and 31. Even after
70 hours dialysis the inhibitor could not be removed from the enzyme.
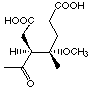
Three compounds of this series rac-25, rac-26
et 29 showed competitive behaviour. Using lower concentrations
of inhibitor and higher concentrations of ALA these compounds activated
the enzyme. The different plots of the inhibition test allowed to recognize
this unusual behaviour clearly. Testing of the two racemic analogues of
the intermediate postulated by Shemin showed that the difference between
the inhibition constants of the two diastereoisomers was only a factor
of 1.5. This difference of inhibition potencity has been more visible when
these compounds where tested with PBGS from Rhodobacter spheroïdes.
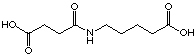
The introduction of a nitro group in 34 compared to 32 improves
slightly the Ki value. It was difficult to estimate
correctly the inhibition constants because the compound 34 showed
an activator effect (activation in the same conditions as rac-25, rac-26
and 29). The replacement of the carboxylate group by a nitro
group in general decreases the Ki value. However the
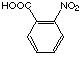
2-nitro benzoic acid (20) shows a considerably increased Ki
value compared to phthalic acid (19).
|
Cx
|
Structure
|
|
Ki
|
Type of inhibition
|
|
C4
|
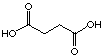
|
16
|
12'500 µM
|
competitive
|
|
C4
|
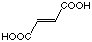
|
17
|
10'700 µM
|
competitive
|
|
C4
|
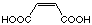
|
18
|
11'500 µM
|
competitive
|
|
C4
|
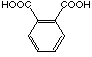
|
19
|
12'500 µM
|
competitive
|
|
C4
|
|
20
|
26'000 µM
|
competitive
|
|
C5
|
|
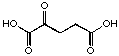
21
|
8'450 µM
|
competitive
|
|
C6
|
|
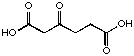
22
|
10'400 µM
|
competitive
|
|
C7
|
|
23
|
12'400 µM
|
competitive
|
|
C7
|
|
24
|
8'600 µM
|
competitive
|
|
C7
|
|
rac-25
|
17'000 µM
|
competitive/
activator
|
|
C7
|

|
rac-26
|
11'900 µM
|
competitive/
activator
|
|
C8
|
|
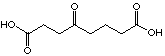
27
|
82 µM
|
uncompetitive
|
|
C9
|
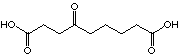
|
28
|
449 µM
|
uncompetitive
|
|
C10
|
|
29
|
7'975 µM
|
competitive/
activator
|
|
C10
|

|
30
|
(-)
|
irreversible
|
|
C10
|
|
31
|
(-)
|
irreversible
|
|
C10
|
|
32
|
22'937 µM
|
competitive
|
|
C10
|
|
33
|
8'331 µM
|
competitive
|
|
C10
|
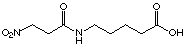
|
34
|
18'175 mM
|
competitive/
activator
|
|
C10
|
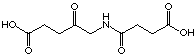
|
35
|
(-)
|
irreversible
|
Table 2: Results of the inhibition tests
Continue
to next chapter
Go to main menu

![]()
![]()




![]()










![]()
