Cx
Structure
Ki
Type of inhibition
C5
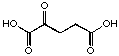
21
8'450 mM
competitive
C6
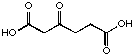
22
10'400 mM
competitive
C7
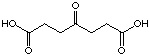
24
8'600 mM
competitive
C8
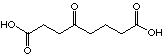
27
82 mM
uncompetitive
C9
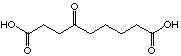
28
449 mM
uncompetitive
C10
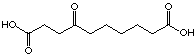
30
(-)
irreversible
Three fundamentally different mechanisms have been proposed for PBGS [5,12,14]. The major difference is the sequence of recognition for the first ALA (A-site or P-site) and the sequence of the reaction which forms the connection between the two substrates. In two proposals, the C-C bond formation is postulated to be the first step obtained via an aldol reaction. In the third proposal, the imine formation between amino group of the P-side substrate and the carbonyl group of the A-side ALA is assumed to be the first step linking the two substrates together. Until now no formal proof in favour or against one of the proposals has been reported.
Analysing our results, the irreversible inhibition of three C10 dicarboxylic acids is remarkable. Compounds 30, 31 and 35 are structurally similar in the two following points: they are dicarboxylic acids separated by eight atoms and in contrast to the other C10 analogues, they contain a keto function and not a amide function (e.g. 32). These compounds seem to be the only inhibitors of this series able to interact so strongly that they are bound irreversibly with the enzyme. They have not the normal characteristics of irreversible inhibitors studied so far in our research group. The epoxydes synthesised by Maurus Marty [58] were found without surprise to be irreversible, but the structures of the molecules in these three compounds are completely different.
|
Cx |
Structure |
Ki |
Type of inhibition |
||||
|
C5 |
|
21 |
8'450 mM |
competitive |
|||
|
C6 |
|
22 |
10'400 mM |
competitive |
|||
|
C7 |
|
24 |
8'600 mM |
competitive |
|||
|
C8 |
|
27 |
82 mM |
uncompetitive |
|||
|
C9 |
|
28 |
449 mM |
uncompetitive |
|||
|
C10 |
|
30 |
(-) |
irreversible |
|||
Table 2: results of the series concerning the extension of the chain length
If we compare just the series of the diacids containing a keto function (Table 2), we can see a regular change in the behaviour of these compounds with the extension of the chain length connecting the two diacids. The first derivatives 21, 22, 23 inhibit competitively the enzyme and we propose that they are recognised as analogues of the substrate in the A Site.
For the C8 analogue, 4-oxo octanedioic acid (27), an uncompetitive inhibition is observed. This seems to indicate that the interaction inhibitor-enzyme is not anymore just at the A-site but at both sites simultanously. We can imagine that the chain is long enough to allow interaction at the two sites, this means that at the same time recognition of the two acid functions and formation of the Schiff's base could occur. This hypothesis of a better fixation of the inhibitor when the length of the chain increases is supported by the following results. The result of the inhibition study with 4-oxo nonadioic acid (28) shows an uncompetitive inhibitor with comparable inhibition potency. At the moment we can not unequivocally attribute the slight increase at the Ki-value. The compound 4-oxo-sebacic acid (30), C10 analogue inhibits irreversibly the enzyme. This last inhibitor, which has the optimal length and all the important functions necessary for his recognition, can be closely fixed to the enzyme. In summary: if the chain is too short the diacids are recognised like analogues of the substrate, if the chain is just a little bit shorter than necessary for the optimal interaction, the inhibitor interacts at the two sites and we are in presence of uncompetitive inhibitors, and finally, if the chain has an optimal length, the compound inhibits the enzyme irreversibly. To complete this series, it would be interesting to test the C11 diacid as well.
The most important result of this series is the significant difference of inhibition between the 4-oxo pimelic acid (24) and the 4-oxo sebacic (30) acid which are structurally similar to the intermediate of Shemin and the intermediate of Jordan. The introduction of the keto function from pimelic acid to obtain 4-oxo pimelic acid (24), shows just a moderate improvement of the recognition. In the case of the sebacic acid, we observe a considerable change from a good competitive inhibitor to an irreversible inhibitor. The introduction of the carbonyl group creates a molecule which is strongly recognised so that it becomes an irreversible inhibitor. In addition, the analogues of Shemin's intermediate rac-25 and rac-26 didn't give spectacular results. The following conclusion can be drawn: the studies of keto diacids, especially the dependence of the inhibition behaviour on the chain length, is a strong argument in favour of Jordan's first mechanism. Finally the following remarks have to be underlined: the worst inhibitors of this series (32 with Ki=22'935 mM and 34 with Ki=18'175 mM) are the diacids with only an amide function (without supplementary ketone). More surprising is the result obtained with 33, a competitive inhibitor with a Ki value of 8'331 mM, close to the Ki of the sebacic acid. The introduction of the CO-NH-NH-CO group didn't affect the inhibition, in comparison to the sebacic acid. The change of the functionality at the end of the chain, exchange of one carboxylate to a nitro group, improves the Ki value slightly .
If we analyse the structure of the analogue of the intermediate reported by Leeper [36], the compound would fullfill the two conditions necessary to obtain an irreversible inhibitor. But Leeper didn't see any inhibition effect on PBGS from Baccillus Subtilus and it would be interesting to test this compound with the PBGS from Escherichia coli.
We tested a series of carboxylic diacids to improve our knownledge of the recognition of intermediate analogues by the enzyme. According to our results, different remarks can be underlined:
- New irreversible inhibitors of the E.Coli PBGS were found. These inhibitors are carboxylic diacids seperated by eight atoms which contain at least one keto function.
- To study more instensively this class of keto diacid inhibitors, variation of the chain length between the two diacid functions was investigated and a regular change was observed. With the extension of the chain, the inhibition type which was first competitive (for the C5, C6, C7 analogues) becomes uncompetitive (C8 and C9 analogues) and finally irreversible (C10).
- To complete this study, inhibition tests of compounds like the keto diacid C11 analogue are necessary. It would be interesting to test C10 compounds with more structural similarities to the postulated intermediates.
- the studies of keto diacids, especially the dependence of the inhibition behaviour on the chain length, is a strong argument in favour of Jordan's first mechanism.
We thank professor P. Schürmann from the Biochemistry Department (University of Neuchâtel) to give us the possibity to do dialysis in his laboratories. We thank the Swiss National Science Foundation and the Foundation of the Basel Chemical Industry to support our work.
[1] A.R. Battersby, C.J.R. Fookes, G.W.J. Matcham, E. McDonald, Nature 1980, 285, 17.
[2] D. Shemin, Methods in Enzymology 1970, XVII Part A, 205.
[3] A.M. Cheh, J.B. Neilands, 'The d-Aminolevulinate Dehydratases: Molecular and Environmetal Properities', in 'Structure and Bonding', 1976, p. 123-170.
[4] F.J. Leeper, Natural Product Reports 1989, 171.
[5] P.M. Jordan, 'The biosynthesis of 5-aminolaevulinic acid and its transformation into uroporphyrinogen III', in 'Biosynthesis of Tetrapyrroles', Ed. P.M. Jordan, Elsevier, Amsterdam, 1991, p. 1-66.
[6] B. Franck, H. Stratmann, Heterocycles 1981, 15, 919.
[7] E.K. Jaffe, H.L. Carrell, J.P. Glusker, L. Shimoni, L.J. Keefe, C. Afshar, M. Volin, Acta Cryst. 1996, D52, 419.
[8] P.T. Erskine, N. Senior, S. Awan, R. Lambert, G. Lewis, I.J. Tickle, M. Sarwar, P. Spencer, P. Thomas, M.J. Warren, P.M. Shoolingin-Jordan, S.P. Wood, J. Cooper, Nature struct. biol.1997, 4, 1025.
[9] H.A. Jackson, 'Pyrroles', in 'Comprensive Organic Chemistry', Ed. D. Barton and D. Ollis, Pergamon Press, Oxford, 1997, p. 275-321.
[10] G. Ksander, G. Bold, R. Lattmann, C. Lehmann, T. Früh, Y.-B. Xiang, K. Inomata, H.-P. Buser, J. Schreiber, E. Zass, A. Eschenmoser, Helv.Chim.Acta 1987, 70, 1115.
[11] A. Eschenmoser, Angew.Chem. 1988, 100, 5.
[12] D. Shemin, D.L. Nandi, J.Biol.Chem. 1968, 243, 1236.
[13] A.R. Chaperon, T.M. Engeloch, R. Neier, Angew.Chem. 1998, 110(3), 369.
[14] P.M. Jordan, J.S. Seehra, J.Chem.Soc.,Chem.Commun. 1980, 240.
[15] R.Neier, 'Chemical synthesis of Porphobilinogen and studies of its biosynthesis', in 'Advances in Nitrogen Heterocycles', JAI Press Inc., Greenwich, 1996, p. 35-146.
[16] E.K. Jaffe, Journal of Bioenergetics and Biomembranes 1995, 27, 169.
[17] Y. Echelard, J. Dymetryszyn, M. Drolet, A. Sasarman, Mol Gen Genet 1988, 214, 503.
[18] A.J. Dent, D. Beyersmann, C. Block, S.S. Hasnain, Biochemistry 1990, 29, 7822.
[19] P.N.B. Gibbs, P.M. Jordan, Biochem.J. 1986, 236, 447.
[20] L.W. Mitchell, E.K. Jaffe, Archives of Biochemistry and Biophysics 1993, 300, 169.
[21] E.K. Jaffe, W.R. Abrams, H.X. Kaempfen, K.A. Harris, Biochemistry 1992, 31, 2113.
[22] E.K. Jaffe, Comments Inorg.Chem. 1993, 15, 67.
[23] N. Senior, K. Brocklehurst, J.B. Cooper, S.P. Wood, P.T. Erskine, P.M. Shoolingin-Jordan, P. Thomas, M.J. Warren, Biochem.J. 1996, 320, 401.
[24] E.K. Jaffe, R.M. Petrovich, S. Litwin, J.Biol.Chem. 1996, 271, 8692.
[25] E.K. Jaffe, M. Volin, C.B. Myers, W.R. Abrams, Biochemistry 1994, 33, 11554.
[26] E.K. Jaffe, A. Shafinaz, L.W. Mitchell, K.M. Taylor, M. Volin, G.D. Markham, Biochemistry 1995, 34, 244.
[27] Z. Zaman, P.M. Jordan, M. Akhtar, Biochem.J. 1973, 135, 257.
[28] P.M. Anderson, R.J. Desnick, J.Biol.Chem. 1979, 254, 6924.
[29] D.W. Christianson, W.N. Lipscomb, Proc.Natl.Acad.Sci.USA 1986, 83, 7568.
[30] E.K. Jaffe, G.D. Markham, Biochemistry 1987, 26, 4258.
[31] E.K. Jaffe, G.D. Markham, Biochemistry 1988, 27, 4475.
[32] E.K. Jaffe, G.D. Markham, J.S. Rajagopalan, Biochemistry 1990, 29, 8345.
[33] R. Neier, R. Lüönd, J. Walker, J.Org.Chem. 1992, 57, 5005.
[34] M. Henz, 'Inhibitionsstudien zum Mechanismus der Porphobilinogen Synthase isoliert aus Escherichia coli CR 261', thèse, Université de Neuchâtel, 1997.
[35] K-M. Cheung, P. Spencer, M.P. Timko, P.M. Shoolingin-Jordan, S. Udenfriend, Biochemistry 1997, 36, 1148.
[36] D. Appleton, A.B. Duguid, S-K. Lee, Y-J. Ha, H-J. Ha, F.J. Leeper, J.Chem.Soc.Perkin Trans.1 1998, 89.
[37] F.J. Leeper, D. Appleton, J.Chem.Soc.,Chem.Commun. 1996, 303.
[38] P. Nayar, N.J. Stolowich, A.I. Scott, Biorg.Med.Chem.Lett. 1995, 5, 201.
[39] A.Cornish-Bowden and C.W. Wharton, 'Reactions of two substrates', in 'Enzyme Kinetics', Ed. D. Rickwood D. Male, IRL Press, Oxford, 1990, p.25-33.
[40] M. Michaelis, M.L. Menten, Biochem.Z. 1913, 49, 333.
[41] S. Granick, D. Mauzerall, J.Biol.Chem. 1958, 232, 1119.
[42] D.P. Tschudy, R.A. Hess, B.C. Frykholm, J.Biol.Chem. 1981, 256, 9915.
[43] P.S. Ebert, R.A. Hess, B.C. Frykholm, D.P. Tschudy, Biochem.Biophys.Res.Commun. 1979, 88, 1382.
[44] P.J. Brumm, H.C. Friedmann, Biochem.Biophys.Res.Commun. 1981, 102, 854.
[45] B. Lindblad, S. Lindstedt, G. Steen, Proc.Natl.Acad.Sci.USA 1977, 74, 4641.
[46] H.A. Sancovich, A.M. Ferramola, C. Battle, M. Grinstein, Methods in Enzymology 1919, XVII Part A, 220.
[47] R. Lüönd, 'Untersuchungen Zum Enzymmechanismus der d-Aminolävulinsäure-Dehydratase aus Rhodopseudomonas spheroides', Inaugural-Dissertation,Universität Fribourg, 1991.
[48] H. Bertschy, 'Pyrrole über die gekreuzte Aldolreaktion', Inaugural-dissertation, Universität Freiburg, 1991.
[49] H. Bertschy, A. Meunier, R. Neier, Angew.Chem. 1990, 102, 828.
[50] J. Heller, A. Jogen, A.S. Dreiding, Helv.Chim.Acta 1972, 55, 1003.
[51] K. Kostova, M. Hesse, Helv.Chim.Acta 1984, 67, 1725.
[52] A. Zürcher, M. Hesse, Helv.Chim.Acta 1987, 70, 1937.
[53] E. Lücke, S. Hünig, Chem.Ber. 1959, 92, 652.
[54] O. Diels, K. Alder, Justus Liebigs Ann.Chem. 1931, 486, 211.
[55] Wakunaga Pharmaceutical Co., Ldt.,Japan, JP 59164797 172 840917
[56] H. Feuer, G.B. Bachman, J.P. Kispersky, J.Am.Chem.Soc. 1951, 73, 4716.
[57] A. Neuberger, J.J. Scott, J.Chem.Soc.,Chem.Commun. 1954, 1820.
[58] M. Marty, 'Synthese von enantiomerenreinen Analoga eines möglichen Zwischenproduktes der PBG-Biosynthese', Thèse, Université de Neuchâtel, 1995.