| [Related articles/posters: 122 037 027 ] |
Among the pesticides which are commonly employed in order to control plant growth, sulfonylureas are widely used. 1 Their site of action was pinpointed to the enzyme acetolactate synthase (ALS). Inhibition of this enzyme, which is needed for the biosynthesis of the essential amino acids valine, leucine and isoleucine, results in rapid cessation of growth and eventual plant death. The absence of ALS in man and other animals helps to explain the low toxicity of the sulfonylureas. Crops, rice and other cereals are able to deactivate these compounds, quickly converting them into inactive metabolites. Their low toxicity, combined with the very low application rates of these compounds, makes them particularly attractive from an environmental and human health standpoint. 2
Owing to the wide interest for sulfonylureas, a theoretical study of these products was undertaken, in order to explain and to investigate their behaviour, and a particular attention was deserved to the hydrolysis mechanism.
Our first goal was to elucidate and to explain the degradation mechanism, and the compounds examined are Chlorsulfuron, Sulfmeturon methyl, Primisulfuron, Rimsulfuron and Bensulfuron methyl.
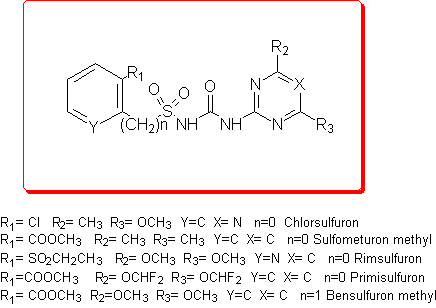
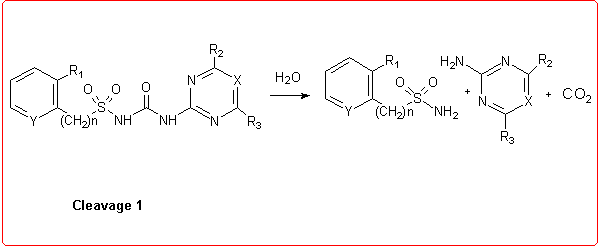
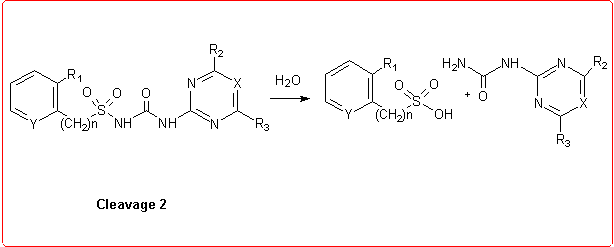
Thus we employed semiempirical quantomechanical methods (AM1 and PM3)3 in order to calculate DHf values for all the products involved in the hydrolytic process. Then, the DH reaction values for these cleavage reactions were obtained and the calculation results were in good agreement with the experimental data.
Eventually, we studied the hydrolysis of Rimsulfuron performed under basic conditions,4 which follows a different pathway with respect to the other sulfonylureas:
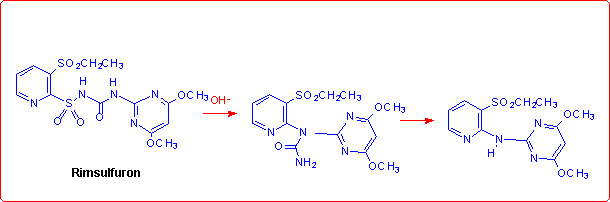
This unusual behaviour was explained by supposing that the reaction begins with the formation of the corresponding anion species, as reported in the figure below.
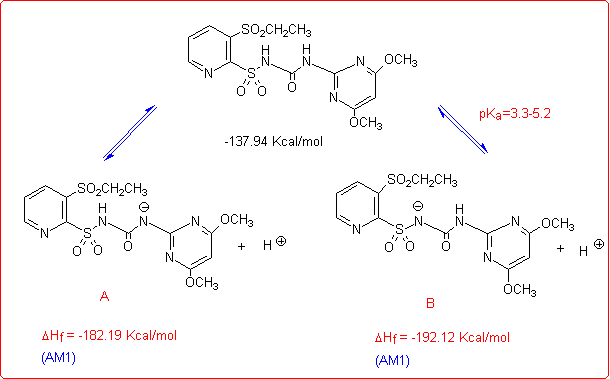
The anions A and B could give an intramolecular nucleophilic substitution reaction (SNAr) that can proceed through either a five- or a three-membered transition state TS1 and TS2, both leading to the cleavage of the C-S bond. From AM1 calculations5 the five-membered transition state (TS1) resulted to be favoured over the three membered one (TS2), owing to both its energy and the correct symmetry and orientation of the frontier molecular orbitals involved.
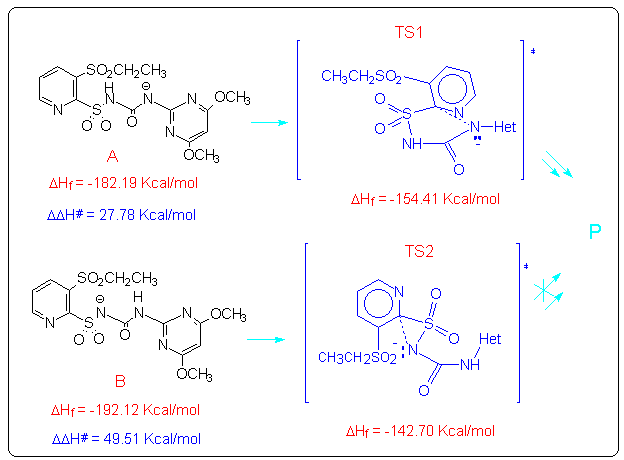
By using the semiempirical method AM15, we calculated the geometry and the energy of the transition states TS1 and TS2. Moreover we localized the coordinate reaction for the two processes that correspond with the only one normal mode with immaginary frequency that is -565.53 cm-1 for TS1 and -345.34 cm-1 for TS2. This coordinate reaction consists in a syncronous moving of the two centres involved in the reaction, the pyridinic carbon and the deprotonated nitrogen. Then the energy DDH# of the two processes was calculated and it resulted that the first process, which passes through the formation of the five-membered transition state TS1, is favoured over the second one. This result is in agreement with the experimental data because only the first process leads to the formation of the experimentally isolated products.
In order to confirm these results, we studied the form and the distribution of the frontier orbitals HOMO and LUMO both for anion A and B. We found that these frontier orbitals have the distibution and the correct symmetry to allow the SNAr only for anion A. So that, according to the FMO theory6, the first process, passing through TS1, is the only one allowed.
Finally, the frontier electron density was calculated both for anion A and B and the most significative results are reported in table 1 and in table 2.
We can notice that for anion A the donor centre (the site with the biggest value of the frontier electron density for the HOMO) is the deprotonated nitrogen and the acceptor centre (the site with the biggest value of the frontier electron for the LUMO) is the carbon near the sulphonyl group. On the contrary for anion B, the donor site is a carbon atom in the heterocicle ring and the acceptor centre is a carbon in the aromatic ring.
According to the FMO theory6 it results that only the process which passes throught the five-membered ring is allowed while the other process, which passes throught the three-membered ring is forbidden.
Bibliography
1.Levitt G., in Pesticide Chemistry: Human Welfare and the Environment, Vol.1, p. 243, Miymato J. and Kearney P.C., Eds.,Pergamon Press, New York, 1983.
2. Sauers R.F. and Levitt G., in Pesticide Synthesis through Rational Approaches , 21, Magee P.S., Kohn G.K., and Mean J.J., Eds., American Chemical Society, Washington, D.C., 1984.
3. HyperChem 5.02, Chemplus 1.6, Hypercube Inc., Ontario, Canada.
a.Stewart, J.J.P. J.Comp.Chemistry, 10, 209, 1989.
b.Thiel W., Tetrahedron, 44, 7393, 1988.
c..Zerner M.C. Semiempirical Molecular Orbital Methods, p.313-366 in Reviews in Computational Chemistry Vol 2, Edited by Lipkowitz K.B. and Boyd, D.B., VCH Publishers, Inc., New York,1991.
4.Marucchini C., Luigetti R., Pestic.Sci., 50, 1-6,1997.
5. Transition states search methods: two different methods for the location of transition structure are available in HyperChem 5.02 Software Package. The first method is the eienvector-following method, and the second is the synchronous transit method.
Eigenvector following method is described in: Baker, J., J.Comp.Chem., 7,385-395, 1986.
The synchronous transit method is described in: Peng, C., and Schlegel, H.B., Israel Journal of Chemistry, 33, 449-454, 1993.
6. Fleming I., Frontier Orbitals and Organic Chemical Reactions, Wiley and Sons, London, 1976.