N-Alkyl-N-nitrosoureas alkylate DNA via an alkanediazonium ion or a solvent-separated ion pair, and this modification of DNA is associated with the mutagenic, carcinogenic, and antineoplastic activities of the drugs. The yield and the selectivity of this alkylation, however, is extremely low. This is particularly frustrating to elucidate the mechanism of alkylation and sequence specificity. On the other hand, it has been reported that N-heterocyclic compounds possessing aromatic 3 ~ 4-ring systems, such as acridines, proflavins, ellipticines, have high affinity for G-C rich sites or specific sequences of the duplex-DNA.
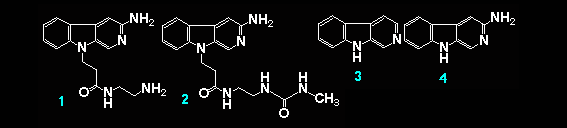
Therefore, if an alkylnitrosourea molecule is linked to one of these intercalators, the yields and specificity of the alkylation should be accelerated by delivery of the alkylater. On the basis of this concept, we have reported a synthesis of an alkylnitrosourea linked to a DNA intercalating methidium structure, and its reaction with DNA.1 As an extention of this study, preparation of a new intercalating molecule, 3-amino-beta-carboline (4), was planed. It has a beta-carboline nucleus (3) that shows higher selectivity for G-C base pair recognition ability than a phenanthridine nucleus does.2 In this paper, we wish to report the synthesis of beta-carboline derivetives (1) and (2) togther with their interaction abilities with calf thymus DNA or a synthesized duplex-DNA oligomer, [5'-d(CATCCCGGGATG)-3']2.

|

|

|