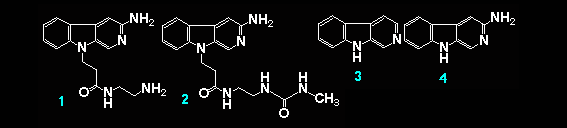
3-Amino-9-N-(2-aminoethyl)carbamoylethyl-beta-carboline (1) was synthesized from 3-methoxycarbonyl-beta-carboline trough successive cyanoethylation, Curtius rearrangement, and addition reaction of ethylenediamine in 45% over-all yield. The terminal aliphatic amino group of (1) was transformed to a N'-methylureido group with succinimidyl N-methylcarbamate to afford the compound (2) in 80% yield. Affinity of (1), (2), beta-carboline (3), and 3-amino-beta-carboline (4) to a DNA oligomer or Calf thymus DNA was spectrometrically studied. Optical spectroscopy of these beta-carbolines showed 25~65 nm bathochromic shifts of the light absorption bands upon binding to the nucleic acids, which suggests binding by intercalation. Schatchard plots, obtained by fluoroescence titration, gave the corresponding binding constants KD to the DNA oligomer; 7.45 x 103, 5.13 x 105, 7.34 x 105, and 7.01 x 105, respectively. The terminal aliphatic amino group considerably decreases the intercalating ability of (1) to DNA.

|

|

|