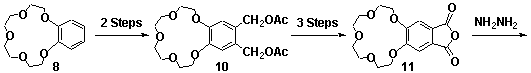The Preparation of 15-Crown-5 Substituted Phthalazinedione and its Application to the Synthesis of Framework-Linked Bis(Crown Ethers)
Clifford M. Jackson,* Ananda S.Amarasekara, Ronald N. Warrener
Centre for Molecular Architecture, Central Queensland University, Rockhampton,
Qld, Australia, 4702.
1,4-Phthalazinediones, prepared via the oxidation of phthalhydrazides, are reactive dienophiles in Diels-Alder reactions. Incorporation of the 1,4-phthalazinedione moiety into a fused [2.2.1]heptane ring system, eg 14, via the Diels-Alder reaction produces an alkene reagent with a planar heterocyclic ring.[1] Starting from the crown ether derivative of 1,4-phthalazinedione 13, new BLOCK 14 has been synthesised, Scheme 1. Application of cyclobutene epoxide methodology (ACE)[2] to this BLOCK has provided easy access to a new series of rigid alicyclic molecules which contain two crown ethers, eg 16, Scheme 2. The geometry of these bis-crown ether molecules is controlled not only by the presence of the planar heterocyclic rings but also the molecule's rigid aliphatic frame(molecular modelling presented).
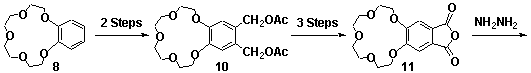
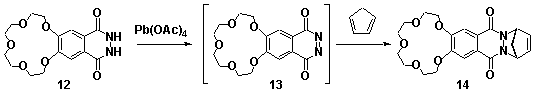
Scheme 1
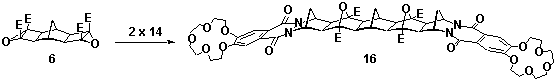
Scheme 2 E=CO2Me
References:
1. Agmon, I.; Kaftory, M.; Nelsen, S. F.; Blackstock, S. C. J. Am. Chem. Soc. 1986, 108, 4477.
2. Warrener, R. N.; Schultz, A. C.; Butler, D. N.; Wang, S.; Mahadevan, I. B.; Russell, R. A. Chem. Commun. 1997, 1023.
View Main Article