| [Related articles/posters: 033 076 120 ] |
Our interest in the synthesis of indoloquinolizines as possible antitumor agents led us to use Teuber's reaction for the preparation of some derivatives incorporating this ring system. During this work, we noticed the lack of 13C NMR data for this class of compounds. Therefore, here we report a 1H and 13C NMR study of several indoloquinolizines synthesized in our group, including the definitive assignments of all the protons and carbons that constitute the indoloquinolizine ring system. We obtained these assignments in DMSO-d6 by means of 2D experiments (COSY, DEPT, HMQC and HMBC).
Starting with tryptamine hydrochloride (4) and acetylacetaldehyde dimethyl acetal we obtain 3-acetyl-2-methyl-7-12-dihydro-6H-indolo[2,3-a]quinolizine chloride (5). Using 5 as starting material, we obtain the corresponding ethyleneglycol ketal (7). Oxidation of 5 with o-chloranil yields a compound with ring C of the tetracyclic system completely aromatized (6). 6 also yields a ketal (8) upon reaction with ethylene glycol.
Treatment of compounds 5-8 with base yields the correponding dihydroindoloquinolizines (9-12), which we will subject to antitumor activity bioassays. Compound 9, derived from 5, shows the interesting behavior of not forming its zwitterion upon treatment with base.
The small group of biogenetically-interesting [1] indole alkaloids that incorporate the zwitterionic indolo[2,3-a]quinolizinium moiety, 1, has recently received some attention in the synthetic field [2] after some of its representatives, such as flavopereirin, 2, and sempervirine, 3, have been described to possess antitumor activity,[3] but the relatively complex preparation methods have hindered the wide availability of analogues.[4]
| 1 | 2 | 3 |
|---|---|---|
For the reader unfamiliar with the indoloquinolizinium skeleton, the following figure illustrates the skeleton itself, as well as its basic naming scheme in rings A, B, C and D. We generated the structure, and its atomic charges, optimizing with PM3. The 2D rendering is missing a negative charge on the indolic nitrogen.
| Highlighting | Display options | Highlighting | Display options |
|---|---|---|---|
| Show A ring | Color atoms by charge | ||
| Show B ring | Show electrostatic potential surface | ||
| Show C ring | |||
| Show D ring | Reset |
Our interest in Teuber's reaction[5] as an easy way to prepare these quinolizinium compounds from tryptamines and (beta)-dicarbonyl compounds, led us to try the synthesis of some derivatives substituted on ring A, by use of the corresponding tryptamines. During this work, we noticed the lack of 13C NMR data for this class of compounds and therefore we reinvestigated the 1H and 13C NMR spectra of 3-acetyl-2-methyl-7,12-dihydro-6H-indolo[2,3-a]quinolizinium chloride obtained from the reaction of tryptamine hydrochloride and the dimethylacetal of acetylacetaldehyde.[6]
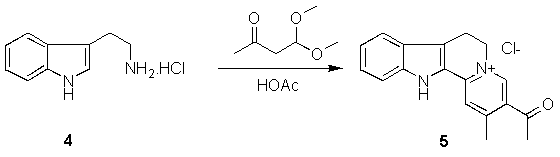
5 was the first compound with an indoloquinolizine ring system synthesized by Teuber. We have obtained some derivatives of 5 (6-12). We report here too a detailed 13C and 1H NMR study of compounds 6-12. The definitive assignments of all protons and carbons were obtained by use of 2D experiments (COSY, DEPT, HMQC and HMBC).