The synthetic methodologies based on the 1,3-dipolar cycloaddition of C-carboxy alkyl nitrones has been furtherly investigated in order to provide a new and direct entry towards nucleoside analogues containing an amino function at the 2â position. The synthetic design, reported in scheme 8, involved the formation of a key intermediate, the tetrahydrofuran acetate 47, which could then be condensed with various nucleoside bases. Our experiments were carried as follows: the initial 1,3-dipolar cycloaddition of nitrone 6 with allyl acetate at 75 ¡C for 48 h afforded an epimeric mixture of 5-substituted isoxazolidines 42 (95 % yield) as exclusive adducts: noteworthy, the 1,3-dipolar cycloaddition proceeded with a good stereoselectivity giving the trans isomer 42a as the major product (3:1 ratio). Treatment of 42a with methyl triflate, followed by catalytic hydrogenation (H2/Pd in methanol), gave the cis 3-dimethylamino-5-hydroxymethyl-tetrahydrofuran-2-ones 44 in 88% yield. After protection of the C5 hydroxyl group with TBDPSCl, direct reduction of 45 with DIBALH (1.2 eq.) provided the epimeric lactols 46; acetylation of 46 led to a readily separable mixture of the acetates 47a and 47b in 62% global yield starting from nitrone 6.
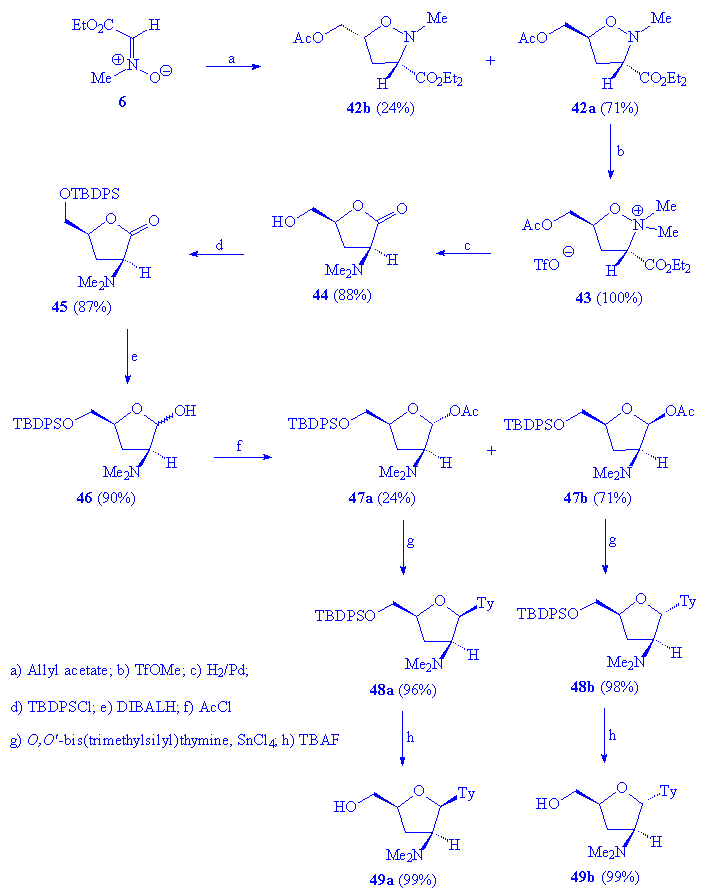
Scheme 8
Compounds 47a and 47b were obtained in a 1:3 relative ratio; the respective configurations were determined by 1H NMR NOE experiments. Thus, whereas 47b showed NOE correlation between H1 and H4, no NOE effects between the same protons have been observed for 47a.
We next explored the coupling reaction with silylated thymine: nucleosidation,
which occurred in high yields (96-98%), was carried out in dichloromethane
at 0 ¡C in the presence of SnCl4 as catalyst and proceeded with
a very high stereoselectivity to afford nearly exclusively a single isomer,
i.e. 48a (b-isomer) from 47a and
48b (a-isomer) from 47b: the 1H
nmr of the crude reaction mixture reveals only traces (~ 1:40 relative ratio)
of the other stereoisomer. Finally, deprotection of compounds 48 with
TBAF gave the modified 2â,3â-dideoxynucleosides 49 in a quantitative
yield. The stereochemistry of thymine analogs 48 and 49 was
determined by NOE experiments, using the unequivocal configuration of the
H4â proton as reference.
The generality of the synthetic scheme
has also been tested by the preparation of the 2â-methyl analog, starting
from C-methyl-C-ethoxycarbonylnitrone 50 (Scheme 9).
The reaction with allyl acetate gave rise to a 1:1.2 mixture of adducts 51a
and 51b, which following the above procedure were transformed in compounds
52.
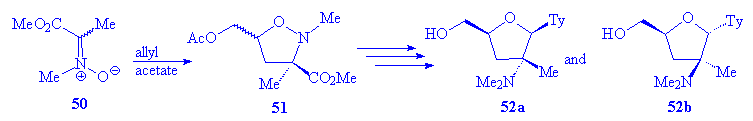
Scheme 9
This method may be applicated to other pyrimidine and purines nucleosides to synthesize the corresponding 2-aminoribonucleoside analogs.
![]() Synthesis of 2â-dimethylamino-2â,3â-dideoxynucleosides
Synthesis of 2â-dimethylamino-2â,3â-dideoxynucleosides