From flatland to prochiral metalation. Aiming for new synthetic methodologies for aromatics and heteroaromatics
Victor Snieckus
The Guelph-Waterloo Centre for Graduate Work in Chemistry, University
of Waterloo, Waterloo, Ontario, N2L 3G1, Canada
This cyperspace multilogue will concentrate on recent developments from our
laboratories in a) extension of directed remote metalation (DreM)
protocol for the regioselective synthesis of dibenzophosphorinones; b)
anionic homo-Fries rearrangements for the construction of benzofuranones;
c) sparteine-induced enantioselective lateral metalation of
ortho-ethyl aryl O-carbamates and d) sparteine-mediated
enantioselective metalation of diverse ferrocenes with planar chirality.
Updates of these areas are given schematically below and questions are raised
for discussion.
(a) Regiospecific and general route to dibenzo[b,e]phosphorinones via anionic
Friedel-Crafts and remote Fries rearrangement synthetic equivalents
As a rational extension of our DreM
approach to thioxanthenone dioxides,1 we have prepared a variety of
the analogous dibenzo[b,e]phosphorinones,2 a heretofore sparsely
investigated class of compounds. (Scheme 1).
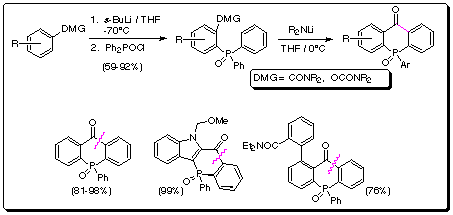
Scheme 1
ortho-Protected O-carbamoyl substrates undergo LDA-induced
remote ring-to-ring carbamoyl migration followed by concomitant regioselective
anionic cyclization to substituted phenyl dibenzo[b,e]-phosphorinones in
moderate to good yield (Scheme 2).
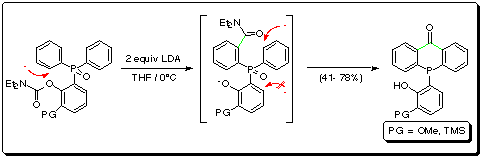
Scheme 2
Of particular note is the double anionic-Friedel-Crafts equivalent to
construct the pentacyclic phosphine oxide (Scheme 3), a possible
starting point for novel ligand preparation. The biological profile of these
systems is largely unknown. The dibenzophosphepinones, analogues of
dibenzazepin antidepressents are also conveniently obtained.
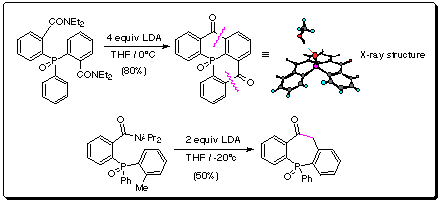
Scheme 3
A carboxylate precursor (Scheme 4), prepared by two different
routes, could be smoothly cyclized to give the parent tricycle in 75% yield,
thereby enhancing the practical nature of this new chemistry.
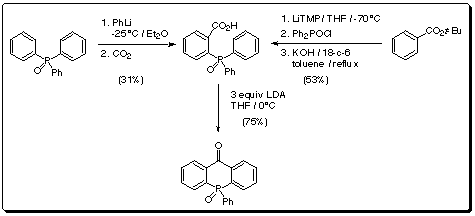
Scheme 4
(b) Anionic homo-Fries rearrangements for the construction of
benzofuranones
In 1983, the anionic equivalent of the ortho-Fries rearrangement
of aryl O-carbamates was discovered in the context of their directed ortho
metalation (DoM) chemistry.3 Recently, we have found an LDA-promoted
O -> C carbamoyl transposition of methyl/benzyl-phenyl-O-carbamates,
methylnapthyl-O-carbamates, and methylpyridyl-O-carbamates to provide a
regiospecific and general route to hydroxyarylacetamides; this transposition
reaction, in turn, permits an equally comprehensive new synthesis of
2(3H)-benzofuranones, naphthofuranones and 2(3H)-furopyridinones.(Scheme
5).
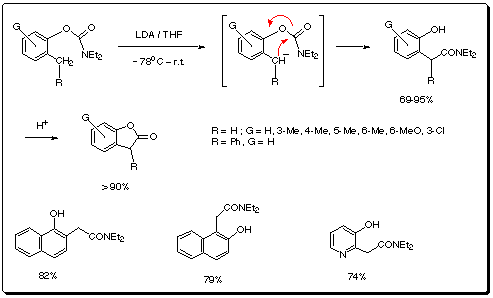
Scheme 5
(c) Sparteine-induced enantioselective lateral metalation of ortho
-ethyl aryl O-carbamates
Stimulated by the results of Beak4 and Hoppe5
demonstrating that (-)-sparteine is an effective ligand for high asymmetric
induction in lithiation-substitution reactions, we have initiated lateral
metalation studies on 1-3 (Scheme 6).
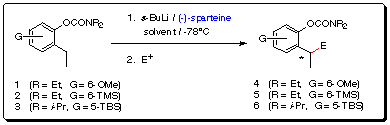
Scheme 6
The 6-substituent in 1 and 2 is required to avoid competitive
aryl C-H deprotonation.3a The 5-TBS carbamate 3 is designed
to prevent 6-deprotonation (Scheme 7).
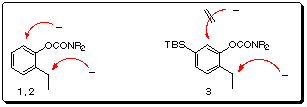
Scheme 7
Optimal conditions for lateral lithiation for carbamates 1 and
2 have been established (Scheme 8). Table 1 summarizes
results of our current studies.

Scheme 8
Table 1 BusLi / (-)-sparteine-induced metalation of O-carbamate
2. Electrophiles, yields and enantioselectivities
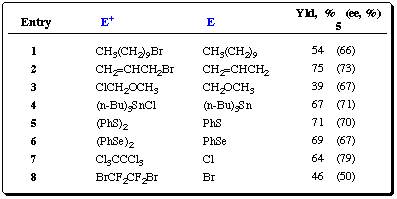
A significant enantioenrichment based on remote silicon
functionalization (3) predicted by molecular modelling and correlated
with aryl C-O bond rotational barriers has been achieved (Figure 1)
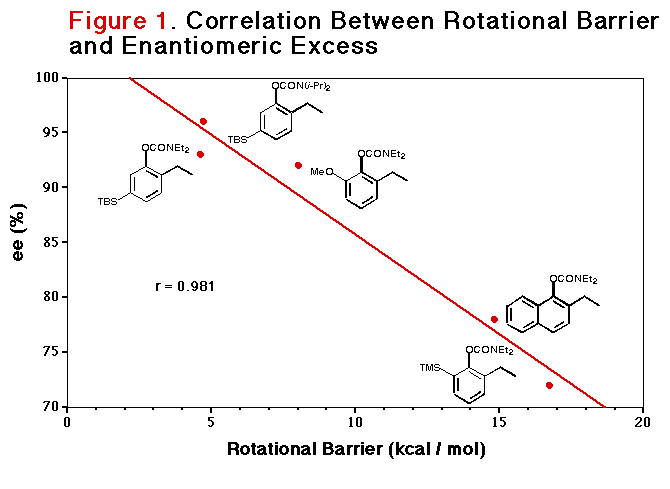
Encouraged by these results, carbamate 3 was comprehensively studied by
variation of reaction parameters and the optimum conditions were applied for
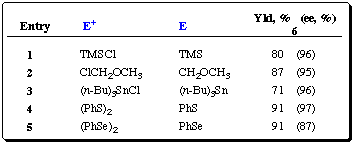
generalization. The results are given in Scheme 9 and Table 2.

Scheme 9
Table 2 BusLi / (-)-sparteine-induced metalation of carbamate
3. Electrophiles, yields and enantioselectivities
(d) Sparteine-mediated enantioselective metalation of diverse ferrocenes with
planar chirality
Ferrocene derivatives with planar chirality6a are of increasing
significance in areas of asymmetric catalysis,6b enantioselective
synthesis,6c and materials science.6d Methods for
diastereoselective preparation of chiral ferrocenes using chiral directed
metalation groups (DMGs) have been accumulating.7 In early 1996, we
reported the first highly enantioselective synthesis of ferrocene derivatives
with planar chirality by way of (-)-sparteine-mediated directed ortho
metalation (DoM) of N,N-diisopropylferrocene-carboxamide
(7).8
Almost simultaneously, Uemura reported
N,N,N',N'-tetramethyl-1R,2R-cyclohexane-diamine as a
chiral ligand in dimethylaminomethylferrocene metalation.9
Using the (-)-sparteine-induced metalation procedure we have prepared a
variety of chiral ferrocenes 8 in excellent yield and with high
enantioselectivity (Scheme 10) and (Table 3).
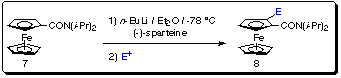
Scheme 10
Table 3 BuLi / (-)-sparteine induced metalation of
N,N-diisopropyl ferrocenecarboxamide 7. Electrophiles, yields and
enantioselectivities

The absolute configuration was established by single-crystal X-ray
crystallographic analysis (Figures 2(a) and 2(b)).
We are currently investigating the use of the (-)-sparteine/BuLi complex in
other ferrocene-DMG systems, e.g. the diethyl carbamate (-OCONEt2) (9)
and diphenylphosphine oxide (-POPh2) (13). Preliminary metalation
results of carbamate 9 are shown in Scheme 11 and Table
4.
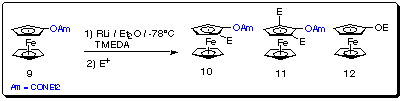
Scheme 11
Table 4 Preliminary metalation results of carbamate 9
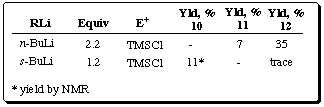
Preliminary metalation results of ferrocenylphosphine oxide 13 are
specified in Scheme 12 and Table 5.

Scheme 12
Table 5 Metalation of ferrocenylphosphine oxide 13
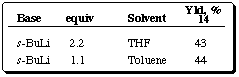
Work in the design and synthesis of new chiral ferrocene derivatives and their
application in catalysis is under active investigation.
- Beaulieu, F.; Snieckus, V. J. Org. Chem. 1994, 59,
6508.
- Gray, M.; Chapell, B. J.; Taylor, N. J.; Snieckus, V. Angew Chem.
1996, in press.
- (a) Sibi, M.P., Snieckus, V., J. Org. Chem., 1983, 48, 1935;
(b) Snieckus, V., Chem. Rev., 1990, 90, 879.
-
(a) Thayumanavan, S.; Lee, S.; Liu, C.; Beak, P. J. Am. Chem. Soc.
1994, 116, 9755; (b) Beak, P.; Kerrick, S.T.; Wu, S.; Chu,
J. J. Am. Chem. Soc. 1994, 116, 3231 and references cited
therein.
-
Hoppe, D.; Hintze, F.; Tebben, P.; Paetow, M.; Ahrens, H.; Schwerdtfeger,
J.; Sommerfeld, P.; Haller, J.; Guarnieri, W.; Kolczewski, S.; Hense, T.;
Hoppe, I. Pure Appl. Chem. 1994, 66, 1479.
-
(a) Togni, A.; Hayashi, T.; Eds. Ferrocenes: Homogeneous Catalysis,
Organic Synthesis, Materials Science ; VCH: Weinheim, 1995; (b)
Sawamura, M.; Ito, Y. Chem. Rev. 1992, 92, 857; (c)
Nicolosi, G.; Patti, A.; Morrone, R.; Piattelli, M. Tetrahedron:
Asymmetry 1994, 5, 1639; (d) Lion-Dagan, M.; Marx-Tibbon, S.;
Katz, E.; Willner, I. Angew. Chem., Int. Ed. Engl. 1995,
34, 1604.
-
(a) Marquarding, D.; Klusacek, H.; Gokel, G.; Hoffmann, P.; Ugi, I. J.
Am. Chem. Soc. 1970, 92, 5389; (b) Rebière, F.;
Riant, O.; Ricard, L.; Kagan, H.B. Angew. Chem. Int. Ed. Engl.
1993, 32, 568; (c) Riant, O.; Samuel, O.; Kagan, H.B. J. Am.
Chem. Soc. 1993, 115, 5835; (d) Richards, C.J.; Damalidis,
T.; Hibbs, D.E.; Hursthouse, M.B. Synlett 1995, 74; (e)
Nishibayashi, Y.; Uemura, S. Synlett 1995, 79; (f) Sammakia, T.;
Latham, H.A.; Schaad, D.R. J. Org. Chem. 1995, 60, 10.
-
Tsukazaki, M.; Tinkl, M.; Roglans, A.; Chapell, B.J.; Taylor, N.J.;
Snieckus, V. J. Am. Chem. Soc. 1996, 118, 685.
-
Nishibayashi, Y.; Arikawa, Y.; Ohe, K.; Uemura, S. J. Org. Chem.
1996, 61, 1172.

















