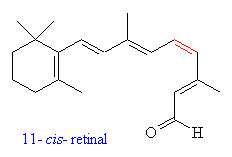
Retinal is the key molecule involved in vision. In fact, there are two different isomers of retinal responsible for converting the energy in light photons into electrical impulses in the retina, 11-cis-retinal and all-trans-retinal. The number 11 refers to the important double bond at the 11th carbon (shown in red) numbering from the end with the ring.

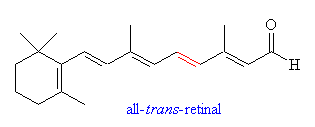
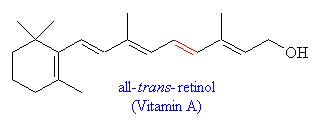
The precursor of 11-cis-retinal is the alcohol all-trans-retinol, commonly known as Vitamin A. This molecule cannot be synthsised by mammals and has to be acquired through the diet. Precursors to Vitamin A are carotenes, which are found in many vegetables including carrots. Perhaps this leads credence to the old superstition that carrots help you see better in the dark - indeed, it is known that a deficiency of Vitamin A leads to night blindness and eventually damage to the retinal cells involved in vision.
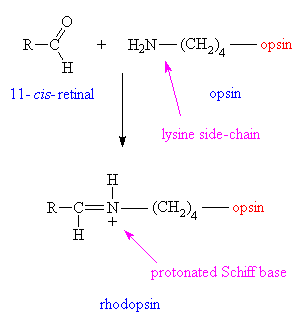
The photosensitive molecule involved in vision is called rhodopsin, (also known as visual purple) which consists of a large protein (having a molecular weight of around 38,000) called opsin, joined to 11-cis-retinal via a protonated Schiff base on one of its lysine side-chains.
Opsin does not absorb visible light, but when it is bonded with 11-cis-retinal to form rhodopsin, the new molecule has a very broad absorption band in the visible region of the spectrum. The peak of the absorption is around 500 nm, which matches the output of the sun closely. When a photon of light falls onto rhodopsin, the molecule absorbs the energy and the cis-double-bond between C-11 and C-12 in the retinal is temporarily converted into a single bond. This means the molecule can now rotate around this bond, which it does by swivelling through 180°. The double bond then reforms and locks the molecule back into position in a trans configuration. Thus the light has isomerised the molecule from cis to trans, and as it did so, it changed the shape of the retinal from curved to straight. Essentially, the energy in a photon has been converted into atomic motion.
 Whereas the 11-cis-retinal fitted into the opsin binding site perfectly, all-trans-retinal is the wrong shape. The Schiff base linkage becomes unstable, and the molecule undergoes a series of shape changes to try and better fit the binding site, before eventually breaking free of the opsin altogether. These rapid movements of the retinal are tranfered to the protein, and from there into the lipid membrane and nerve cells to which it is attached. This generates nerve impulses which travel along the optic nerve to the brain, and we perceive them as visual signals - sight. The free all-trans-retinal is then converted back into the cis form by a series of enzyme-catalysed reactions, whereupon is reattaches to another opsin ready for the next photon to begin the process again.
Whereas the 11-cis-retinal fitted into the opsin binding site perfectly, all-trans-retinal is the wrong shape. The Schiff base linkage becomes unstable, and the molecule undergoes a series of shape changes to try and better fit the binding site, before eventually breaking free of the opsin altogether. These rapid movements of the retinal are tranfered to the protein, and from there into the lipid membrane and nerve cells to which it is attached. This generates nerve impulses which travel along the optic nerve to the brain, and we perceive them as visual signals - sight. The free all-trans-retinal is then converted back into the cis form by a series of enzyme-catalysed reactions, whereupon is reattaches to another opsin ready for the next photon to begin the process again.
In order to see colour, we have 3 types of visual receptors, sensitive to red, green and blue light, but the primary molecule involved in all of these is still 11-cis-retinal. The different wavelength sensitivities are due to small variations in sidegroups attached to the opsin protein.
 But retinal is even more universal than that. There are only 3 types of eyes that can resolve images, those found in molluscs, arthropods and vertebrates. The 3 kinds of eyes are anatomically very different and probably evolved independently. However they all utilise 11-cis-retinal as their light receptor molecule. There are many reasons for this. Firstly, 11-cis-retinal is extremely sensitive to light, and absorbs it very strongly. Moreover, this absorbtion can be readily shifted into the visible region of the spectrum. Secondly, in the absence of light, the molecule is very stable, i.e. it does not isomerise in the dark. If this were not true, we would be constantly plagued by false images. Third, the structural change produced by isomerisation is relatively large (the Schiff base attachment moves over 7 Å in relation to the ring when the molecule isomerises from cis to trans), sufficient to generate a nerve impulse. And finally, the precursors to 11-cis-retinal, the carotenes, are readily available in various edible plants.
But retinal is even more universal than that. There are only 3 types of eyes that can resolve images, those found in molluscs, arthropods and vertebrates. The 3 kinds of eyes are anatomically very different and probably evolved independently. However they all utilise 11-cis-retinal as their light receptor molecule. There are many reasons for this. Firstly, 11-cis-retinal is extremely sensitive to light, and absorbs it very strongly. Moreover, this absorbtion can be readily shifted into the visible region of the spectrum. Secondly, in the absence of light, the molecule is very stable, i.e. it does not isomerise in the dark. If this were not true, we would be constantly plagued by false images. Third, the structural change produced by isomerisation is relatively large (the Schiff base attachment moves over 7 Å in relation to the ring when the molecule isomerises from cis to trans), sufficient to generate a nerve impulse. And finally, the precursors to 11-cis-retinal, the carotenes, are readily available in various edible plants.