 |  | 
|
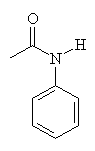
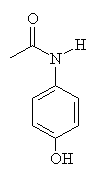
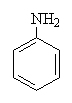
| Acetanilide | Paracetamol | Aniline |
|---|
The painkilling properties of paracetamol were discovered by accident when a similar molecule (acetanilide) was added to a patient's prescription about 100 years ago. But since acetanilide is toxic in moderate doses, chemists modified its structure to try and find a compound that was less harmful but which still retained the analgesic properties. One of these compounds is N-acetyl-para-aminophenol, which is also known as acetaminophen in the US and paracetamol (from para-acetyl-amino-phenol) in the UK. When mixed with codeine it goes by the tradename Tylenol.
 |  | 
|
| Acetanilide | Paracetamol | Aniline |
|---|
In fact, in the body, the original compound, acetanilide is partially converted into a mixture of paracetamol and aniline. The paracetamol provides the painkilling properties, but the aniline is toxic. Paracetamol has a very similar structure to aspirin, and because of this they are recognised by the same enzyme. This enzyme is responsible for the biosynthesis of prostoglandins, which are involved in the dilation of blood vessels that causes the pain experienced in a headache. Reduction of the amount of prostoglandin, therefore, helps prevent headaches and other pain.
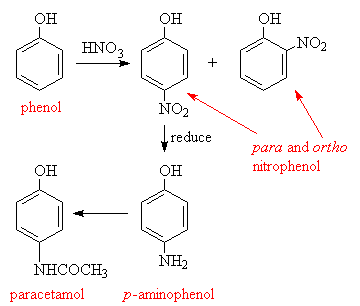
Paracetamol is one of the most common drugs used in the world, and is manufactured in huge quantities. The starting material for the commercial manufacture of paracetamol is phenol, which is nitrated to give a mixture of the ortho and para-nitrotoluene. The o-isomer is removed by steam distillation, and the p-nitro group reduced to a p-amino group. This is then acetylated to give paracetamol.
Because paracetamol is a potent drug that is available without prescription, it is often used in suicide attempts, and in this respect it is potentially more dangerous than other over-the-counter drugs such as aspirin. This is because paracetamol overdoses often cause liver failure, and there have been many cases where attempted suicides have awakened from an overdose and changed their minds, yet still died a few days later from liver damage.
The reasons for this poisoning are to do with the process by which paracetamol is eliminated from the body. It is first metabolised to a quinone imine:
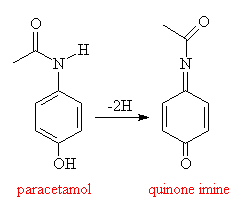
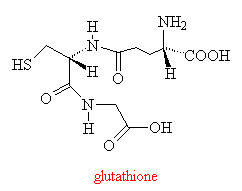
This compound is extremely toxic, and like other such compounds is eliminated in the liver by reaction with a tripeptide, glutathione. If insufficient glutathione is available, the toxic quinone will not be eliminated and begins to react with cellular proteins and nucleic acids in the liver, eventually causing irreparable damage.
|
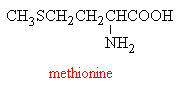 | 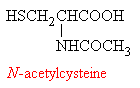
|
However, two compounds, methionine and N-acetylcysteine can boost levels of the vital glutathione in the liver, and so can be used as antidotes for paracetamol poisoning if the overdose is discovered in time. A new formulation of paracetamol is now being marketed in the UK which incorporates methianine, such that the drug carries its own antidote with it!