Ozone (from the Greek ozein "to smell") is a blue, pungent gas that is created whenever an electric discharge is passed through oxygen. It is often smelt after lightning, or near electrical equipment, or near the ocean, and is responsible for the familiar bracing smell of the seaside. Pure ozone can be obtained by fractional distillation of O2-O3 mixtures, and ozone can be condensed to a deep blue, highly explosive liquid. This is not a reaction for the faint hearted, as it is notoriously temperamental and explosions occur readily and without warning!
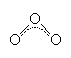
The O3 molecule is bent, with an O-O-O bond angle of 117°. The bond length in ozone (1.28Å) is intermediate between the single bond found in hydrogen peroxide HO-OH (1.49Å) and the double bond in oxygen O=O (1.21Å). From this, it is apparent that the bonding in O3 must have considerable double bond character, and so perhaps a good descrition of its bonding is:

As well as an electrical discharge, ozone can be created by the action of high energy, ultra-violet light upon oxygen. The UV splits the O2 into 2 oxygen atoms, which then strike other O2 molecules to form ozone. This process occurs high up (30 km above the ground) in the Earth's atmosphere, in a layer called the ozone layer. This layer is not very dense and is only about 20 km thick. The ozone that is produced here can absorb yet more UV, and fall apart. Thus, both of these two processes, the creation and destruction of ozone in the upper atmosphere, serve to absorb almost all of the ultra-violet radiation that reaches the Earth and prevent it reaching the planet surface. Ultra-violet radiation is dangerous, since it can cause serious radiation burns (sun-burn) to unprotected living organisms, as well as damage the DNA present in their cells, creating mutant genes and leading to skin cancers. Thus the ozone layer acts as a barrier around the Earth protecting all the living organisms on the surface below. It is interesting to note that the amount of ozone that performs this vital task is quite small; if all of it were collected and compressed to a pressure of 1 atmosphere at the Earth's surface, it would form a layer only 3 mm thick!
 | The ozone layer forms a barrier around the Earth, protecting it from harmful UV radiation |
Recently is was discovered that chlorofluorocarbons (CFCs), which are used as the coolants in refrigerators and propellants in aerosols, can disturb the delicate chemical balance occurring in the ozone layer. In effect, via a series of chemical reactions, the chlorine atoms liberated from CFCs soak up oxygen atoms and prevent them reacting with O2 to form ozone.
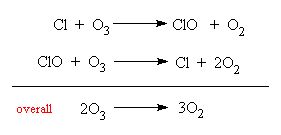
This depletion of ozone has led to an increasing amount of ultra-violet light reaching the Earth's surface in the past 10 years, and has been accompanied by a corresponding increase in the reported number of skin cancers. This is especially true in polar regions, where the prevailing weather patterns, the cold temperatures in the stratosphere (-80°C) and high level icy clouds, concentrate this ozone destruction effect. At certain times of the year, especially in September and October, the ozone layer almost completely disappears in the Antarctic for a period of few months. The discovery of this ozone destruction mechanism not only earned the three chemists involved (Paul Crutzen, Mario Molina and Sherwood Rowland) the Nobel prize for Chemistry in 1995, but led to a United Nations protocol on the protection of the ozone layer, signed in Montreal in 1987. The use of CFCs has since been drastically reduced. CFCs are now banned from aerosols, and replacements for their use in refrigeration are now being introduced. However CFCs are so tenacious, that it will take many years before they are all removed from the atmosphere. Some estimates say that the ozone layer will not re-establish itself properly for 100 years.
Although it is not poisonous, inhaling ozone in moderate quantities is unpleasant and causes coughing and chest pains, and has been linked to asthma. Various nitrogen and carbon oxides which are produced by combustion, especially in car exhausts, can react with oxygen in the presence of sunlight to produce smog. One by-product of these reactions is ozone, and on calm days during summer, this ozone can build up close to the ground in cities, causing numerous respiritory problems among pedestrians. Hence the use of catalytic converters in modern cars, as an attempt to reduce the emission levels of these oxides. This low-lying ozone, being very chemically reactive, can also attack the chemical bonds in a number of materials, particularly rubber, and has been linked to the premature weathering of buildings, paintwork and statues.
Another instance where you might encounter ozone directly is by flying in an aircraft close to the ozone layer, say at altitudes of 15 km. Some of the air in an aircraft's cabin is drawn in from outside, and at this altitude the concentration of ozone would rapidly become unpleasant. So the incoming air is passed through filters that catalytically decompose the ozone back to oxygen.