Carbon monoxide, CO, is a colourless toxic gas which is formed when carbon is burned in a deficiency of oxygen. It is also formed when carbon is used as a reducing agent, for example producing phosphorus from phosphate rock. It is also formed in automobile exhausts, released by certain marine plants, and occurs in small quantities naturally in the atmosphere. CO is made commercially along with hydrogen by a process known as steam reforming, in which methane or light petroleums are passed over a nickel catalyst at about 750°C. The mechanism is complex, but the main reaction is:
![]()
Another method is by reduction of carbon dioxide with hydrogen:
![]()
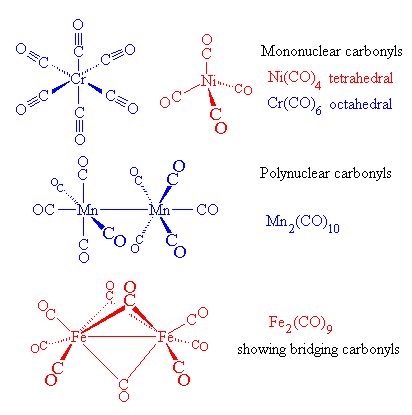
The CO triple bond is the strongest chemical bond known, which makes CO relatively inert, although it can be burnt in oxygen to produce CO2. The most important aspects of its chemistry arise from its ability to react with transition metals using a special type of chemical bond (dp-pp bonding). Direct bonding of CO with transition metals produces metal carbonyls, and carbonyl derivatives are known for all of the transition metals. The first metal carbonyls, Ni(CO)4 and Fe(CO)5 were discovered by A. Mond in 1890 and 1891, which he later developed into a commercial process for the isolation of pure nickel. Metal carbonyls now take many forms and have many different structures, and are extremely important industrially as catalysts.
CO is very important in industry, since it is a precursor to a number of important organic chemicals. A mixture of CO and H2 is called synthesis gas, and is used both for the synthesis of methanol and in the 'hydroformylation reaction', in which a H atom and a formyl group (HCO) are inserted into the double bond of an alkene to form an aldehyde. This can be further reduced to an alcohol. Cobalt compounds are often used as catalysts for this process at temperatures of around 150°C and >200 atmospheres pressure. Several million tonnes of C7-C9 alcohols are produced in this way each year.
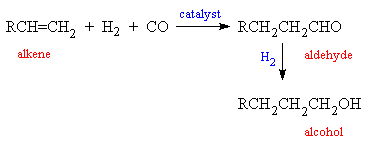
Another important commercial process involving CO is the carbonylation of methanol to give acetic acid using a rhodium catalyst in the presence of iodide ions.
CO forms a very strong bond with the iron atom in hemoglobin in the blood, and once bonded cannot be dislodged (unlike oxygen and CO2 which detach easily and reversibily). This means that as more CO is inhaled, more red blood cells get 'used up' leaving fewer and fewer available to carry the vital oxygen to the muscles, tissues and brain of the animal. If not treated immediately with O2 and possibly a blood transfusion, the animal would die of asphyxiation.